Tucidinostat/Chidamide (Trade name in Mandarin :剋必達®, Trade name in English : Kepida®)
Tucidinostat's first indication in Taiwan – combined with Exemestane for the treatment of HR+/Her-2-advanced breast cancer
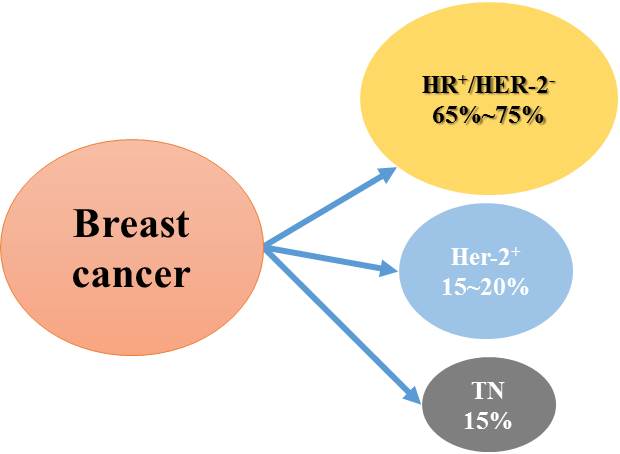
Background of the breast cancer
The incidence of breast cancer is the highest among all cancers in women. It is primarily classified into three types, i.e., HR+, HER-2+, and triple negative breast cancer. HR+ patients account for 65–75% of all cases, the highest proportion among all types. These patients are positive for both estrogen receptor (ER) and progesterone receptor (PR) on the surface of cancer cells, which can usually be stimulated by hormones and undergo accelerated growth. Although endocrine therapy in combination with CDK4/6 inhibitors is effective in halting disease progression in these patients, about half of patients develop disease progression/relapse or resistance after treatment for 24 months. Therefore, the dual resistance to the first-line treatment with endocrine therapy combined with CDK4/6 inhibitor is an important clinical problem and challenge to be solved.
Tucodinostat combined with Exemestane will effectively overcome the dual resistance caused by the first-line use of endocrine therapy plus CDK4/6 inhibitors through a unique epigenetic regulatory mechanism, and provide a new option for second-line therapy.
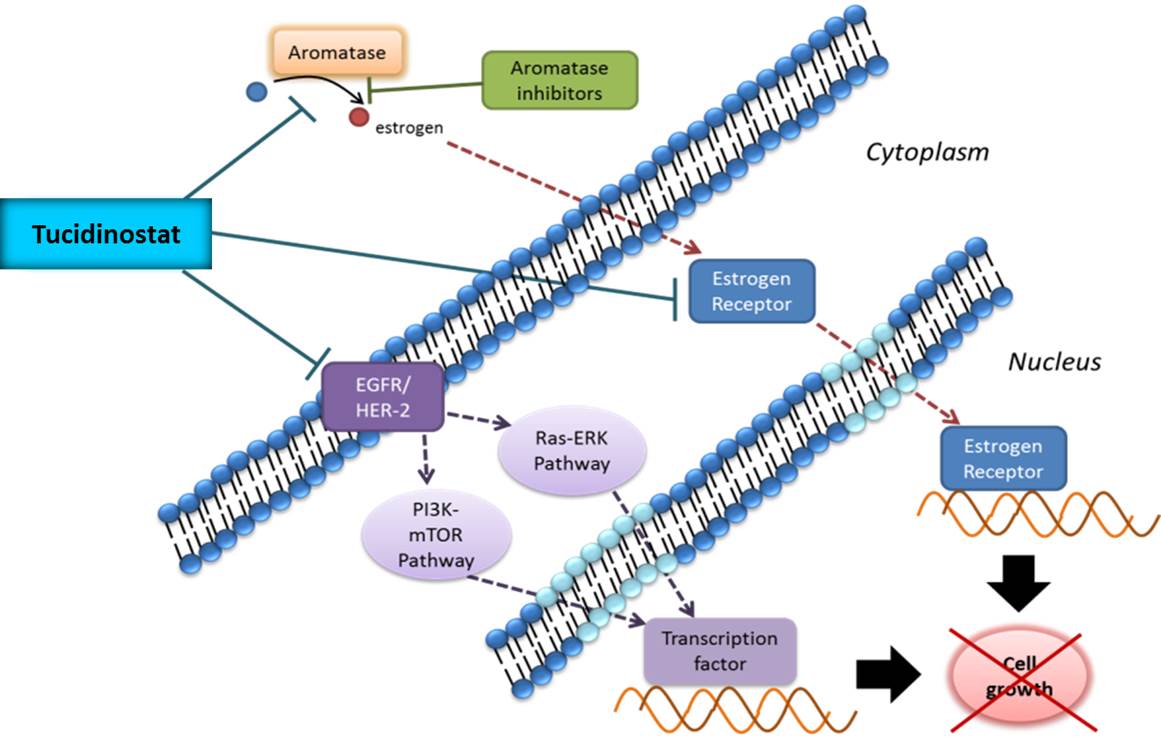
Rationale for the treatment of HR+/Her-2- breast cancer with Tucidinostat
This is mainly based on the following studies: (1) In 2007, G. Sabnis et al. showed that HDACi effectively reduced the resistance of breast cancer cells to AI and increased the sensitivity of the treatment; (2) in 2011, CW Chou et al. indicated that HDACi inhibited the expression of EGFR in colorectal cancer cells; (3) in 2013, BM Müller et al. showed that the higher expression of HDACs 1, 2, and 3 in patients with HR+ breast cancer than in HR- patients (P<0.05) influenced the disease progression, cancer metastasis, and reduced the survival rate. Tucidinostat is a subtype selective HDACi and its anti-cancer effect is mainly exerted by the inhibition of the expression of HDACs 1, 2, 3, and 10. For the treatment of HR+ breast cancer, Tucidinostat reduces the expression of HDACs 1, 2, and 3 in the tumor and has the potential to slow down the disease progression and prolong the survival of patients; (4) Syndax’s (U.S.) HDACi Entinostat (belonging to the benzamide-based) combined with exemestane was used in patients with resistance to the non-steroidal AI or relapsed after the treatment to provide a new treatment opportunity for them. In September 2013, the drug combination received Breakthrough Therapy Designation from FDA and may be able to reverse the induced resistance due to long-term therapy with non-steroidal AI, regain the sensitivity to the drug for slowing disease progression.
For these reasons, Chipscreen Biosciences began an open stage study of clinical phase III trial, in which 20 patients with HR+/Her-2- advanced breast cancer who had failed or relapsed after at least one-line prior endocrine therapy were treated with Tucidinostat in combination with Exemestane. The results showed that the mPFS was 7.6 months (the mPFS for Exemestane alone was about 3 months), the two drugs after combination had no drug-drug interaction, and the safety of adverse reactions was controllable.
GNTbm has partnered with Chipscreen Biosciences to conduct a pivotal phase III clinical trial, enrolling a total of 420 patients with the same inclusion/exclusion criteria in Taiwan and China. Chipscreen Biosciences has announced its successful trial results in China and obtained the NMPA approval for breast cancer in November 2019. GNTbm has conducted the trial in Taiwan at the same time, and also announced a successful result when combined both China & Taiwan data, submitting an application for NDA in Taiwan, and obtaining the first indication approval of Tucidinostat in Taiwan in March 2023. Tucidinostat is the world's first epigenetic modulator approved for the second-line treatment of HR+/Her-2- advanced breast cancer, through a unique epigenetic regulation mechanism which increases the sensitivity of endocrine therapy and reduces the drug resistance.
Proposed mechanisms of Tucidinostat in combination with exemestane for the treatment of HR+/Her-2- breast cancer
Pivotal Phase III Clinical Trial for the Tucidinostat Treatment of HR+/HER-2- Advanced Breast Cancer (CDM301)
The clinical trial, called CDM301, recruited patients with HR+/Her-2- advanced breast cancer who had failed or relapsed with at least one previous endocrine therapy, treated with Tucidinostat plus Exemestane. The patients were stratified according to presence or absence of visceral metastasis, and randomly assigned 2:1 to the experimental group and the placebo group for treatment until disease progression or withdrawal from the trial due to drug toxicity, and the primary endpoint was PFS assessed by clinical investigators. The trial period is about 5 years.
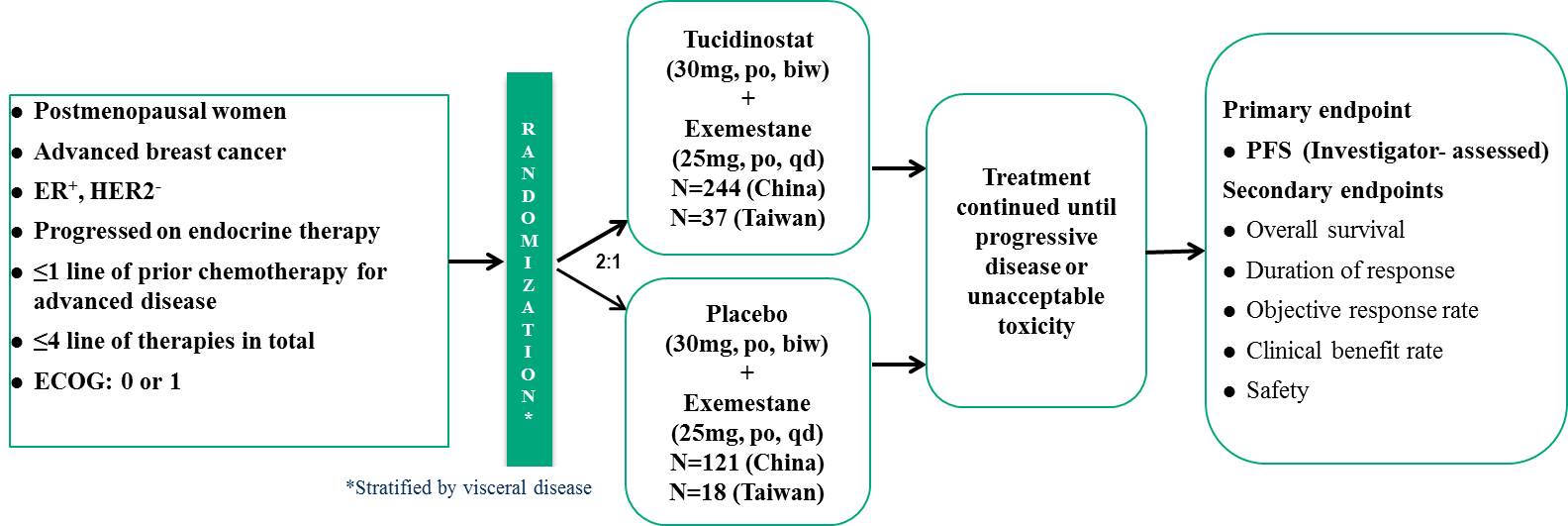
Efficacy assessment
CDM301 is a well-designed, placebo-controlled, randomized, multicenter, pivotal Phase III clinical trial in Taiwan and China. A total of 420 patients were enrolled and randomized 2:1 to Tucidinostat plus Exemestane (experimental group) and placebo plus Exemestane (placebo group), with 281 and 139 patients, respectively. The efficacy outcomes mPFS of the primary endpoint of the trial were 7.4 months and 3.7 months (HR=0.716; 95% CI, 0.562 ~ 0.911; P=0.0066) for the experimental and placebo groups, respectively. Data analysis of 55 patients in Taiwan showed that Tucidinostat combined with Exemestane (experimental group) and placebo plus Exemestane (placebo group) were randomly assigned 2:1, with 37 and 18 cases, respectively. The mPFS of the primary endpoint were 8.6 months and 3.7 months (HR=0.516; 95% CI, 0.268 ~ 0.993; P=0.0477), for the experimental and placebo groups, respectively. In 420 patients, the objective response rate (ORR) of the secondary endpoint efficacy of the trial was 16.73% in the experimental group and 7.91% in the placebo group, which was a significant statistical difference.
Safety data
Common adverse events in the Tucidinostat combined with Exemestane groups were hematologic toxicity and gastrointestinal toxicity, both of which were controllable adverse reactions that could be restored by reducing the dose of investigational drug or suspending the investigational drug.
Tucidinostat meets the medical needs of HR+/Her-2- advanced breast cancer patients as a second-line treatment
According to NCCN treatment guidelines, endocrine therapy combined with CDK4/6 inhibitors is mainly recommended for the standard first-line treatment of HR+/Her-2-advanced breast cancer. In addition, according to Taiwan‘s health insurance reimbursement regulations, HR+/Her-2- advanced breast cancer patients can use CDK4/6 inhibitors for 24 months in health insurance for life. And according to the international literature it has been reported that the first-line treatment of HR+/Her-2- advanced breast cancer with endocrine therapy combined with CDK4/6 inhibitor showed excellent therapeutic benefits with mPFS around 24 months. Based on these facts, it has been suggested that the use of endocrine therapy combined with CDK4/6 inhibitors for the first-line treatment of HR+/Her-2- advanced breast cancer will become dominant in Taiwan.
When the first-line treatment with endocrine therapy combined with CDK4/6 inhibitor fails, patients usually develop drug resistance to both endocrine therapy and CDK4/6 inhibitor, and subsequently the second-line treatment options are limited, mainly due to the occurrence of dual drug. According to the NCCN treatment guidelines, the drugs that can be used for second-line therapy are quite limited, the first recommendation: if the patient has never used any CDK4/6 inhibitor before, CDK4/6 inhibitor combined with endocrine therapy is recommended for second-line therapy; the second recommendation: If the patient has PIK3CA mutation, it can be recommended to use Alpelisib (PI3Ki) combined with fulvestrant; a third recommendation is that patients can use Everolimus (mTORi) in combination with Exemestane.
Tucidinostat (HDACi) combined with Exemestane can be the fourth option. Tucidinostat is an epigenetic modulator that can overcome the dual resistance problems caused by treatment with endocrine therapy and CDK4/6 inhibitor. After launch of Tucidinostat in Taiwan market, GNTbm will conduct a real-world study in Taiwan to evaluate the therapeutic benefit of Tucidinostat plus Exemestane when endocrine therapy combined with CDK4/6 inhibitor fails in the first-line treatment of HR+/Her-2- advanced breast cancer.