Tucidinostat/Chidamide (Trade name in Mandarin :剋必達®, Trade name in English : Kepida®)
Tucidinostat new indications - for the treatment of relapsed/refractory peripheral T-cell lymphoma (R/R PTCL)
R/R PTCL
R/R PTCL is a rare disease, and according to the WHO classification in 2018, there are more than 20 subtypes. R/R PTCL subtypes are heterogeneous and disease progresses aggressively, and there is a lack of standard treatment drugs for many years. Treatment with conventional chemotherapy results in low response rate, poor tolerability, easy to relapse, and 5-year overall survival rate only about 25%.
The US FDA approved Adcetris (brentuximab vedotin) as first-line therapy for PTCL in combination with chemotherapy in 2018, and Adcetris is an ADC (Antibody-drug conjugate) drug. The trial was based on the CHOP chemotherapy regimen for the traditional treatment of PTCL as the control group, and Adcetris (substituted O) + CHP as the experimental group.
The US FDA approved three new drugs for the treatment of R/R PTCL, including Pralatrexate (Folotyn®), Romidepsin (FK228, Istodax®) and Belinostat (PXD101, Beleodaq®) in 2009, 2011 and 2014, respectively. The NMPA approved Tucidinostat for the treatment of R/R PTCL in 2014 in China . The Ministry of Health, Labor and Welfare approved Tucidinostat for the treatment of R/R PTCL in 2021 in Japan.
At present, only Folotyn® has entered the Taiwan market for the treatment of R/R PTCL, and the clinical demand for effective and safe treatment drugs for patients with R/R PTCL in Taiwan has not been met.
Overall evaluation of the effectiveness of Tucidinostat in the treatment of R/R PTCL in pivotal trials in China and Japan
Pivotal clinical data in China
The primary endpoint of Tucidinostat clinical trial for the treatment of R/R PTCL is the objective response rate (ORR), which was used as the efficacy indicator in the other three international pivotal trials of the new drugs approved outside China. In the pivotal phase II clinical trial of Tucidinostat, at a dose of 30 mg twice a week, the clinical investigators assessed the ORR to be 29.1% (23/79), 95% CI: 19.4%~40.4%. Tucidinostat trial in China was designed in accordance with the three international new drug pivotal clinical trials, in addition to the clinical investigators' evaluation of efficacy, an independent review committee (IRC) has been established to independently evaluate the ORR, the main efficacy indicator of the pivotal clinical trial. The ORR of the four new drugs in the treatment of R/R PTCL were 28%, 27%, 25% and 26%, respectively. Tucidinostat has similar ORR when compared with the other three international new drugs.
In addition to the ORR, the results of the Tucidinostat pivotal Phase II clinical trial showed that the 3-month duration of response rate (DORR) in the FAS population was 24.1% (19/79), much higher than Pralatrexate's 12% (13/109), indicating that Tucidinostat may have better efficacy for R/R PTCL than Pralatrexate. Tucidinostat was approved by China NMPA in December 2014.
This is based mainly on the following studies:
(1) In 2007, G. Sabnis et al. showed that HDACi effectively reduced the resistance of breast cancer cells to AI and increased the sensitivity of the treatment;
(2) in 2011, CW Chou et al. indicated that HDACi inhibited the expression of EGFR in colorectal cancer cells;
(3) in 2013, BM Müller et al. showed that the higher expression of HDCA 1, 2, and 3 in patients with HR positive breast cancer than in HR-negative patients (P<0.05) influenced the disease progression, cancer metastasis, and reduced the survival rate. Chidamide is a subtype selective HDACi and its anti-cancer effect is mainly exerted by the inhibition of the expression of HDAC1, 2, 3, and 10. For the treatment of HR positive breast cancer, chidamide reduces the expression of HDCA 1, 2, and 3 in the tumor and has the potential to slow down the disease progression and prolong the survival of patients;
(4) Syndax’s (U.S.) product Entinostat combined with exemestane was used in patients with resistance to the non-steroidal AI or relapsed after the treatment to provide a new treatment opportunity for them. In September 2013, the drug combination received Breakthrough Therapy Designation from FDA and may be able to reverse the induced resistance due to long-term therapy with non-steroidal AI, regain the sensitivity to the drug for the relief of the disease. Entinostat and chidamide are highly similar with respect to the mechanism of drug action and the chemical structure.
Pivotal clinical data in Japan and South Korea
Design of Phase III clinical trial
In pivotal clinical trials in Japan and South Korea with HBI-8000 (Tucidinostat) for the treatment of R/R PTCL, the primary endpoint was objective response rate (ORR), and the ORR evaluation by IOERC (Independent Overall Efficacy Review Committee) was 46%, and the disease control rate was 72%. Among them, the second largest subtype of PTCL, AITL, obtained an ORR of 88%. Tucidinostat was approved by the Ministry of Health, Labor and Welfare on November 30, 2021 in Japan.
Table 1. Comparison in the pathological subtypes recruited in the trials between Tucidinostat and international approved drugs for R/R PTCL (clinical data in China)
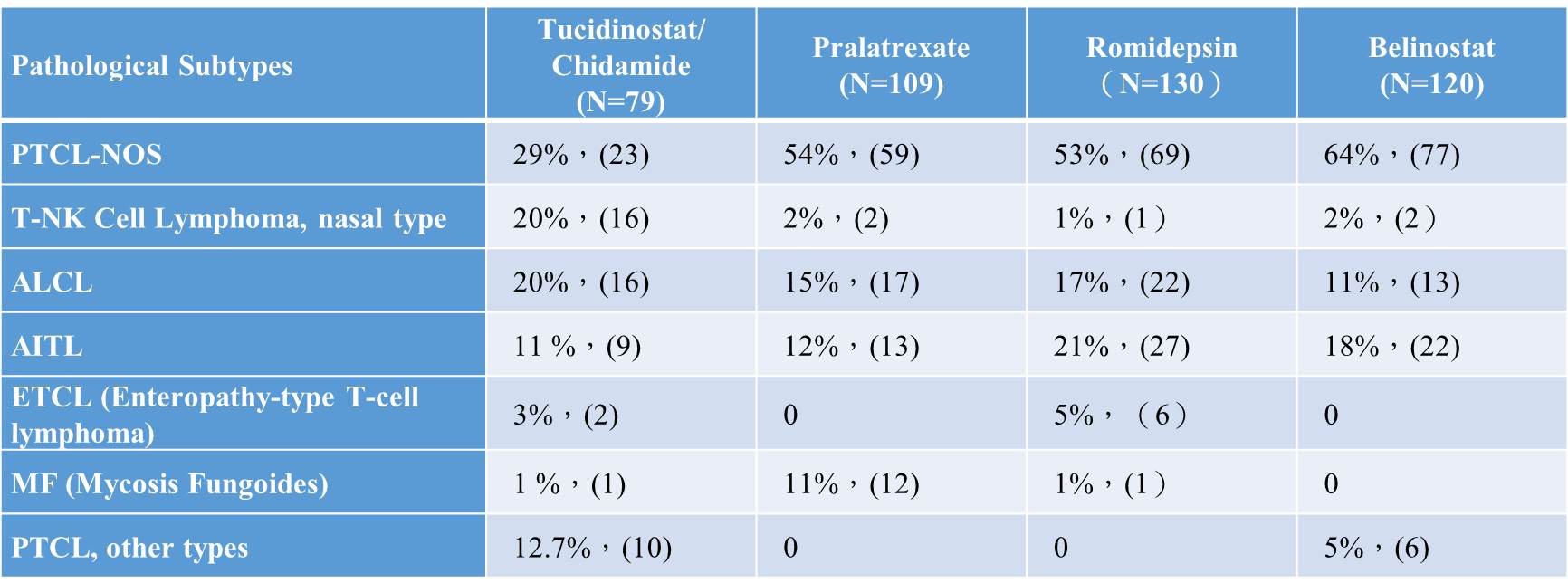
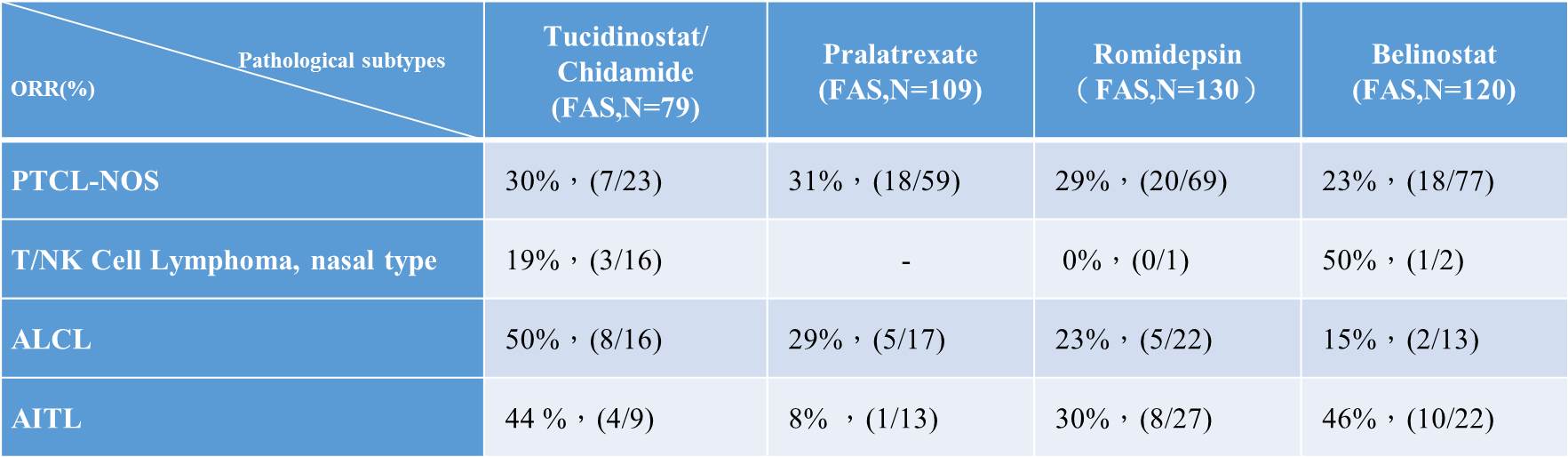
Table 2. Comparison in the response rates of pathological subtypes accounting for >10% of all types of PTLC between Tucidinostat and international approved drugs for R/R PTCL (clinical data in China)
Benefit and Risk Assessment
Tucidinostat is a subtype-selective HDAC inhibitor, and according to the comprehensive results of their respective pivotal clinical trials with R/R PTCL as indication, the comprehensive efficacy evaluation results show that Tucidinostat may have better efficacy (ORR 46% in Japan and South Korea) or at least the same efficacy (ORR 28% in China) than the three international approved drugs in the world.
Convenience and Compliance
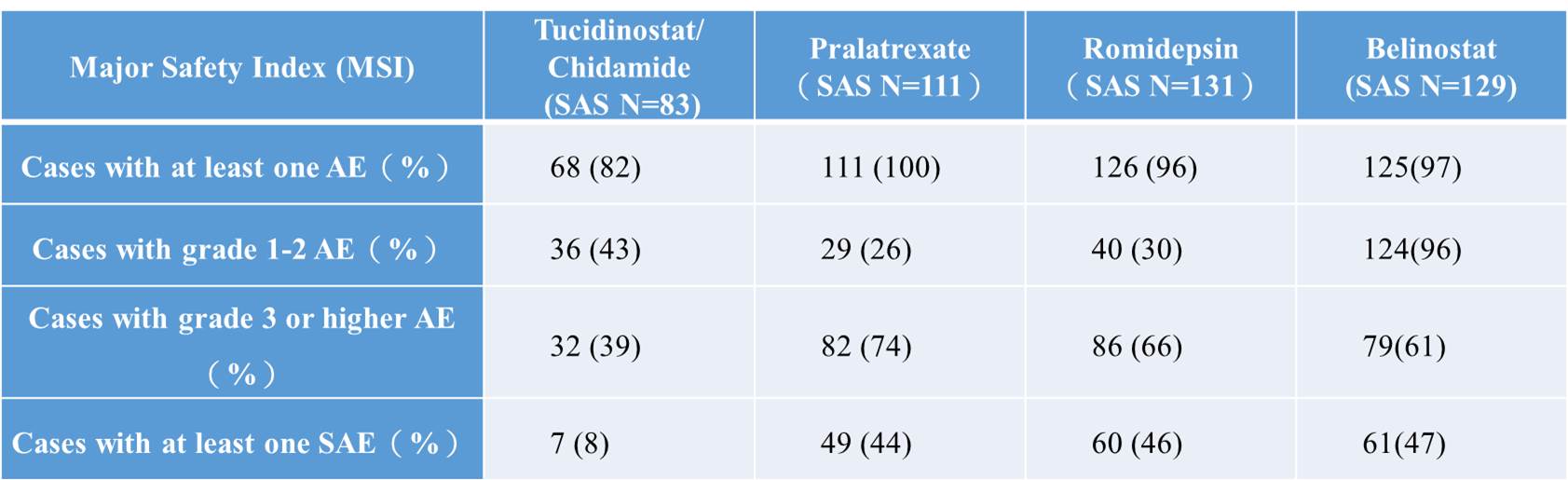
Tucidinostat is orally administered twice per week; compared with the three intravenously administered new drugs marketed overseas, it offers a more convenient administration route, which is expected to have a higher compliance. Even with oral administration, the incidence and severity of adverse events and the severe adverse events (SAE) with digestive system manifestations, such as diarrhea and nausea, were much lower than those of the three intravenously administered drugs. The following table shows the adverse events of the four drugs.
Table 3. Adverse events of Tucidinostat and international approved drugs for R/R PTCL (clinical data in China).
Compassionate Therapy and clinical trial with Tucidinostat for R/R PTCL in Taiwan
In February 2020, Taiwan's TFDA approved Compassionate Therapy for the first time to use Tucidinostat (Taiwan trade name: Kepida®) 5 mg tablet manufactured by GNTbm in Taiwan for the treatment of R/R PTCL. GNTbm has provided compassionate therapy for more than 2 years free of charge, with a total of 36 patients showing treatment benefits.
Among these patients in Taiwan, the main disease subtypes of R/R PTCL are as follows: PTCL-NOS, AITL, NK/T Lymphoma, ALK-/ALK+ ALCL, MEITL, and ATL/L.
According to data from large-scale international studies, the median survival for R/R PTCL is only 5.8 months. Among the patients treated with compassionate therapy in Taiwan, many patients have been treated with Kepida® tablet for more than 1 year, showing significant efficacy with an ORR of about 45%, and safe and controllable adverse events.
Although GNTbm has submitted NDA for the treatment of R/R PTCL, GNTbm will conduct a small clinical trial in Taiwan to comply with the NDA review in accordance with supplementary requirements of TFDA.
Possible new indication for Tucidinostat - combination with R-CHOP for the first-line treatment of MYC/BCL-2 dual expressor diffuse large B-cell lymphoma (DLBCL).
Diffuse large B cell lymphoma (DLBCL) is the most common type in the NHL, accounting for 30 to 40% of adult NHL in Europe and the United States. According to the 2016 Taiwan Cancer Registry, there was a total of 3,426 newly diagnosed patients with malignant lymphoma, of which the NHL accounted for about 2,474 people and 2,160 cases of B-cell lymphoma, while 1,326 cases were large B-cell lymphoma, accounting for about 53.6% of the total NHL, accounting for 38.7% of all malignant lymphomas.
According to the 2016 Taiwan Cancer Registry, 909 new cases of DLBCL over the age of 60 were diagnosed, accounting for 68.5% of the total number of DLBCL patients in that year. About 54.6% of Taiwan's newly diagnosed DLBCL patients were at stage III-IV.
MYC/BCL-2 dual expressor DLBCL accounts for about 30% of all DLBCLs, and the treatment effect of standard therapy R-CHOP is quite limited and prone to relapse. There are no new drugs approved for MYC/BCL-2 dual expressor DLBCL in the world, and the clinical demand is quite urgent.
The current status of treatment for DLBCL
(1) R-CHOP is the standard first-line therapy
As a type of invasive NHL, DLBCL's progression of disease is relatively soon, but a certain percentage of patients can be cured after receiving appropriate treatment. In the past, DLBCL's treatment was mainly CHOP chemotherapy containing doxorubicin, and about one-third of patients survived for more than 5 years after receiving the combined chemotherapy. Rituximab combined with CHOP treatment regimen improves total complete response (CR) and
long-term survival rates for DLBCL patients, so both domestic and international clinical guidelines unanimously recommend Rituximab (R) combined with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP)
regimen as the standard first-line treatment for DLBCL. Unfortunately, about 1/3 of patients still do not respond to first-line R-CHOP therapy or relapse early, and some clinical studies have tried to improve the prognosis of these patients through "R-CHOP + X" combination, but have not been successful so far. Prognostic stratification factors such as Hans classification cannot fully explain the characteristics of this part of the patient population, and the efficacy needs to be improved.
(2) Treatment with R-CHOP for MYC/BCL-2 dual-expressor DLBCL has poor efficacy outcome
The studies have found that some DLBCL patients had overexpression of MYC and BCL-2 proteins, while so-called "dual expressor" lymphoma (DEL) for MYC/BCL-2 simultaneous overexpression. The clinical detection rate reported overseas was 29-45%, significantly higher than the "double-hit" lymphoma (DHL). The clinical detection rate of DEL in China based on small sample size study was 32 to 36%, which may be related to different definition about the detection threshold used in each study. The determination of "dual expressor" is mostly based on the definition for positive by using immunochemical test by following WHO standard: MYC is at least 40%, BCL-2 is at least 50%, and is not accompanied by the re-arrangement of MYC and BCL-2 and/or BCL-6 genes. Taiwan has not yet had a complete large-scale investigation study to analyze the epidemiology of DEL and the ratio of patients. According to interviews with clinicians at National Taiwan University Hospital, it was estimated that the incidence rate of this subtype of patients may be about 20-30%. According to the 2016 Cancer Registry, the newly diagnosed cases of DLBCL in the year was 1,326, and the number of DLBCLs with DEL subtypes may be only about 300 patients. Several hematology oncologist in Taiwan said that although these subtypes account for only 20-30% of DLBCL, the prognosis and response rate of R-CHOP treatment is much lower than that of non-MYC/BCL-2 dual-expressor DLBCL, and clinical unmet needs are not yet solved, and new and effective therapies are still urgently needed.
Rationale for the treatment of MYC/BCL-2 dual-expressor DLBCL with R-CHOP combined with Tucidinostat
Treatment with R-CHOP for dual expressor DLBCL has poor efficacy, because these patients often have clinical pathological characteristics of poor prognosis, including old age, late-stage tumor, multiple sites of lesions, high score of international prognostic index (IPI) and poor treatment response. Although there are currently two new drugs in combination with R-CHOP for treatment of MYC/BCL-2 dual expressor DLBCL patients in Phase III clinical trials, the clinical needs have not been met and there is still an urgent need for new effective drugs to provide effective treatment.
A number of cellular and animal studies of Tucidinostat have been conducted to provide the mechanisms for MYC/BCL-2 dual-expressor DLBCL treatment:
1. Tucidinostat can promote the growth inhibition and to induce doxorubicin-triggered apoptosis of tumor cells by inhibiting the DNA damage repair process.
2. Tucidinostat can induce the CD20 expression of DLBCL tumor cell, thereby enhancing tumor inhibition activity of anti-CD20 monoclonal antibodies (e.g. Rituximab);
3. Tucidinostat inhibits MYC and BCL-2 expression in tumor cells and improves the efficacy of standard treatment for MYC/BCL-2 dual expressor DLBCL.
Through the above different mechanisms, Tucidinostat combined with R-CHOP standard therapy exerts synergistic inhibition effect on DLBCL tumor growth, especially for the treatment of MYC/BCL-2 dual expressor DLBCL.
Tucidinostat can met the clinical need for MYC/BCL-2 dual-expressor DLBCL
Clinical studies have shown that the R-CHOP therapeutic efficacy and prognosis of MYC/BCL-2 dual expressor DLBCL patients were significantly lower than that of non-MYC/BCL-2 dual expressor DLBCL patients, and there is still need for improvement in the R-CHOP-based treatment. There has been no prospective, large-scale clinical study to show successful treatment for MYC/BCL-2 dual expressor DLBCL patients, so exploring a new combination with new drug that is effective and relatively safe for the dual expressor DLBCL patients on the basis of the R-CHOP regimen is a significant and unmet clinical need.
Although there are currently two new drug combinations with R-CHOP for MYC/BCL-2 dual expressor DLBCL in phase III clinical trials, one of which is “Ibrutinib combined with R-CHOP”, showing improvement of mOS, mPFS, and EFS in patients under 60 years of age, but not in patients over 60 years of age. Epidemiological surveys show that nearly 60% of patients with DLBCL disease are over 60 years of age. Another drug was “Lenalidomide + R-CHOP”, which did not met the primary endpoint. Therefore, MYC/BCL-2 dual-expressor DLBCL has a very urgent clinical need.
In July 2022, GNTbm partners (Chipscreen Biosciences) have completed the enrollment of 418 MYC/BCL-2 dual-expressor DLBCL patients, randomly assigned to the experimental group (Tucidinostat + R-CHOP) and the placebo group (placebo + R-CHOP) in a 1:1 manner, and held the IDMC meeting in February 2023. Based on the trial outcomes, IDMC recommended that Chipscreen Biosciences apply for conditional marketing authorization from China Food and Drug Administration (NMPA).
In order to provide the new treatment for MYC/BCL-2 dual-expressor DLBCL patients in Taiwan, GNTbm will do its best to fulfill the requirements for NDA filing in Taiwan according to the TFDA/CDE recommendations, and provide new treatment options to meet the treatment needs of MYC/BCL-2 dual-expressor DLBCL patients.