Overview
GNTbm's core focus for new drug development is on a new drug combination platform that targets TME regulation, featuring two independently developed drug candidates. One is the immune activator GNTbm-38, which mainly regulates gene expression to affect the cell composition and gene expression of the TME, that, in turn, can effectively remodel the TME, converting “cold tumors” into “hot tumors”. It is beneficial to further combine with unique multi-targeted tyrosine kinase inhibitor, immune checkpoint inhibitor (ICI), and anti-PD-1/VEGF bispecific antibody to significantly enhance the efficacy of anti-tumor immunotherapy. The second orally developed new drug candidate is GNTbm-TKI, which primarily is a highly effective selective inhibitor targeting specific tumor immune-regulatory kinases, thereby effectively inhibiting tumor growth, invasion, and metastasis, as well as remodeling the TME. GNTbm-TKI is a potent selective inhibitor of TYRO-3, AXL, c-MER, BTK, ROS1, NTRK2, MET, and VEGFR2. These new chemical and orally administered immune-regulatory drug candidates, with unique innovative mechanisms, can remodel the patient’s TME, activate the patient’s immune system, allowing the immune system to re-recognize the tumor, attract a large number of CTLs into the TME, stimulate attacks on the tumor by CTLs, produce superior anti-tumor immune benefits, and generate lasting immune memory. This is expected to significantly enhance the effectiveness of cancer immunotherapy in patients with advanced cancer.
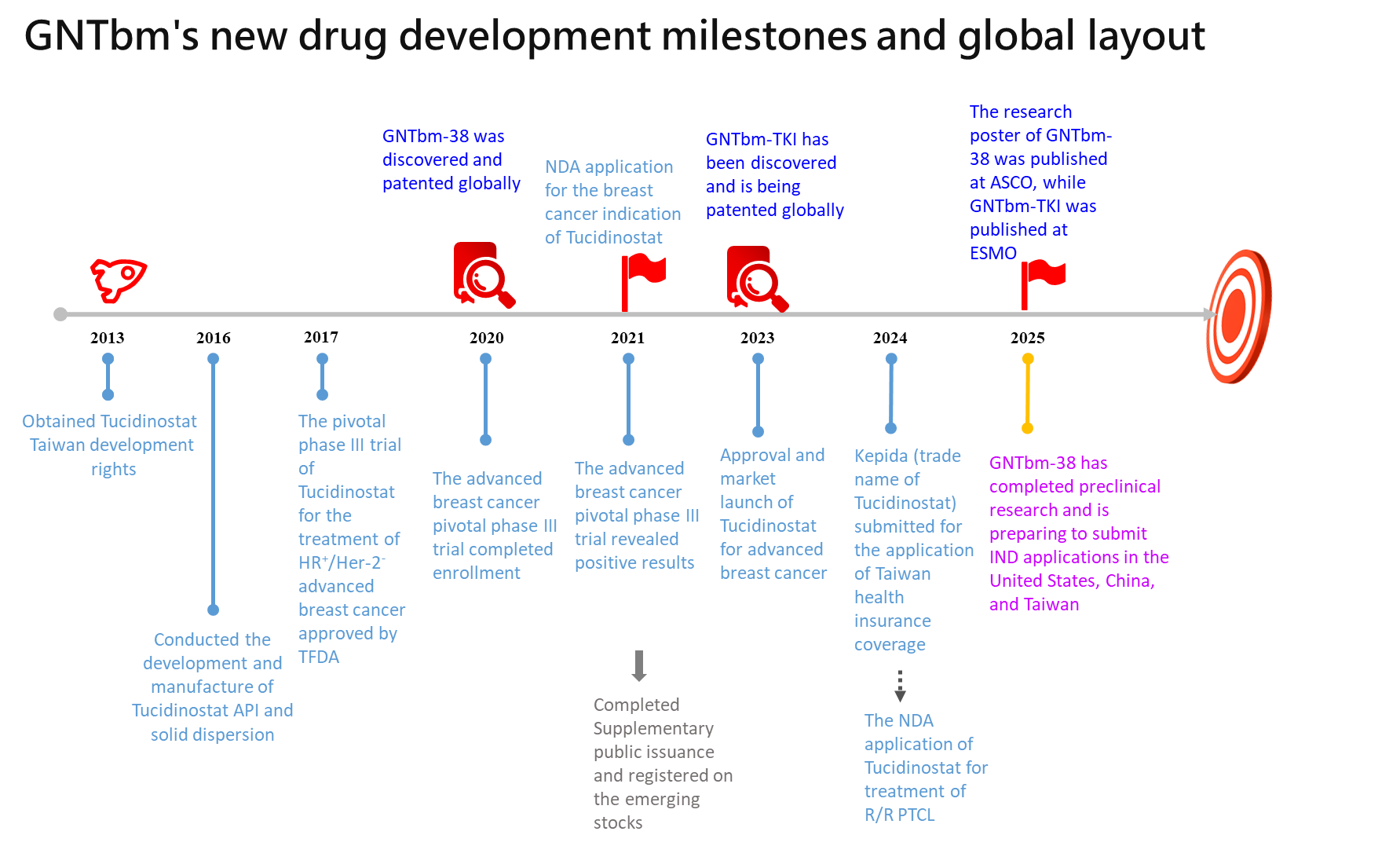
On the other hand, the new epigenetic regulatory drug Tulcidinostat/Chidamide, which has been in-licensed, has obtained its drug approval by TFDA after 10 years of development. It is used for the treatment of HR+/Her-2- advanced breast cancer and has been filed for new indication peripheral T-cell lymphoma.
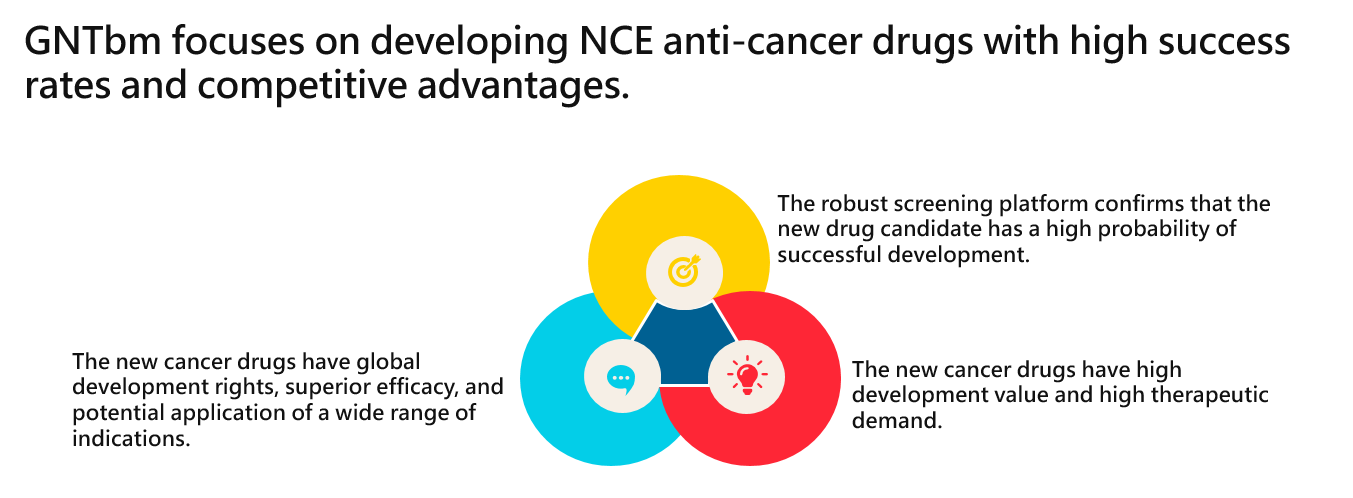
GNTbm independently develops new drugs with new chemical entities for cancer immunotherapy, aiming to meet the clinical unmet needs of patients with advanced cancer, and provides safer and more effective next-generation cancer immunotherapies to satisfy the clinical treatment needs of patients with advanced cancer.

Vision
Cancer is a major life-threatening disease. The goal of GNTbm's new drug development is to meet the treatment needs of advanced cancer patients and provide them with the ultimate opportunity to achieve clinical cure. GNTbm's independent new drug R&D mainly focuses on new chemical entity of oral new drugs with innovative mechanisms for cancer immunotherapy. One approach is to develop a powerful immune activator, which activates the immune system and remodels the TME; the other is to develop a potent immune-regulating multi-kinase inhibitor, which is multi-target kinase inhibitor for cancer immunotherapy. GNTbm is committed to addressing the urgent treatment needs of advanced cancer patients worldwide with a new generation of cancer immunotherapy featuring powerful and innovative anti-cancer mechanisms.
GNTbm continuously innovates and develops new drugs with innovative mechanisms for application in the field of cancer immunotherapy by monotherapy or combination therapy to maximize their effectiveness. This will offer advanced cancer patients a new treatment option that is more effective, has longer-lasting effects, and is safer, thereby enhancing and optimizing the treatment benefits and quality of life for patients, and allowing them and their families to regain beautiful moments in life.
Mission
GNTbm aims to become a pioneer in the field of global cancer treatment through development of oral, effective, and innovative cancer immunotherapy. These new drug candidates are powerful epigenetic immune activators and multi-kinase inhibitors that primarily operate through a unique mechanism to remodel the TME and activate the patient’s immune system. This activation allows cytotoxic T lymphocytes (CTLs) to effectively attack tumors, achieving significant anti-tumor immune benefits and generating lasting immune memory, leading to prolonged disease remission for patients.
GNTbm independently develops new drugs in Taiwan, with patent layouts around the world, is dedicated to developing powerful oral cancer immunotherapy new drugs to help global patients with advanced cancer, and meanwhile secures a place in the global field of cancer immunotherapy.