Potent immune-regulatory multi-tyrosine kinase inhibitor
In the past 20 years, multiple tyrosine kinase inhibitors have been approved for the treatment of cancer and other indications. The GNTbm R&D team focuses on the development of multi-tyrosine kinase inhibitors with strong immune activation, for primary application in cancer immunotherapy. Therefore, the GNTbm R&D team has independently developed a novel immune-regulating multi-tyrosine kinase inhibitor, GNTbm-TKI.
GNTbm-TKI is a small molecule drug candidate that has been selected after years of R&D, displaying novel structure, advantage of oral administration, and excellent immunoregulation activity. Studies on enzyme, cell, and animal test platforms have shown that GNTbm-TKI possesses strong immunoregulatory activity, and when used in combination with specific HDAC inhibitors, it exhibits very superior TME modulation activity, capable of transforming cold tumors into hot tumors and significantly increasing tumor immune response rates. GNTbm-TKI can be used as a monotherapy for the treatment of advanced neuroendocrine tumors, or in combination with HDAC inhibitors, ICIs, and anti-PD-1/VEGF bispecific antibodies for cancer immunotherapy. GNTbm-TKI is a potent selective multiple tyrosine kinase inhibitor that effectively inhibits specific targets, including TYRO-3, AXL, c-MER, BTK, ROS-1, NTRK2, VEGFR2, and MET. GNTbm has completed patent applications in multiple countries worldwide. GNTbm-TKI is one of the key components of drug combinations in cancer immunotherapy, primarily functioning to effectively remodel the TME.
GNTbm-TKI has entered preclinical research and been expected to enter Phase I clinical trial around 2027. It is anticipated that Phase II clinical trial will be conducted in combination with GNTbm-38, which firstly will be applied for orphan drug designation. The strategy is to first obtain drug approval through a single-arm pivotal Phase II trial, and then expand into other new indications. GNTbm-TKI will be a multi-tyrosine kinase inhibitor with strong immunomodulatory effects, primarily applied in combination of 'GNTbm-TKI plus I' for indication expansion, where I represents HDAC inhibitor, ICI, or anti-PD-1/VEGF bispecific antibody, belonging to powerful cancer immunotherapy treatments that are expected to treat various advanced solid tumors.
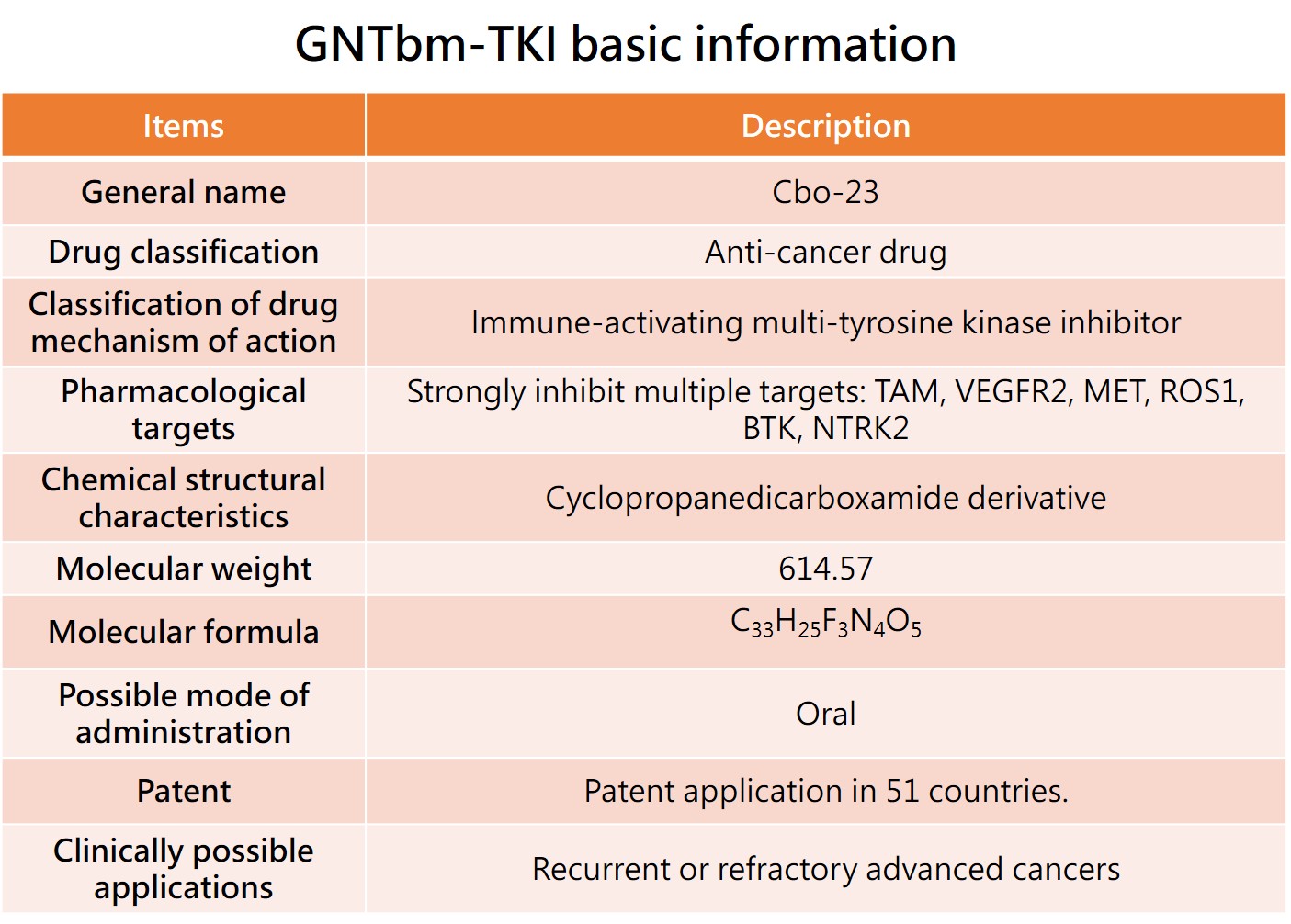
GNTbm-TKI is a small molecule drug candidate that has been selected after years of R&D, displaying novel structure, advantage of oral administration, and excellent immunoregulation activity. Studies on enzyme, cell, and animal test platforms have shown that GNTbm-TKI possesses strong immunoregulatory activity, and when used in combination with specific HDAC inhibitors, it exhibits very superior TME modulation activity, capable of transforming cold tumors into hot tumors and significantly increasing tumor immune response rates. GNTbm-TKI can be used as a monotherapy for the treatment of advanced neuroendocrine tumors, or in combination with HDAC inhibitors, ICIs, and anti-PD-1/VEGF bispecific antibodies for cancer immunotherapy. GNTbm-TKI is a potent selective multiple tyrosine kinase inhibitor that effectively inhibits specific targets, including TYRO-3, AXL, c-MER, BTK, ROS-1, NTRK2, VEGFR2, and MET. GNTbm has completed patent applications in multiple countries worldwide. GNTbm-TKI is one of the key components of drug combinations in cancer immunotherapy, primarily functioning to effectively remodel the TME.
GNTbm-TKI has entered preclinical research and been expected to enter Phase I clinical trial around 2027. It is anticipated that Phase II clinical trial will be conducted in combination with GNTbm-38, which firstly will be applied for orphan drug designation. The strategy is to first obtain drug approval through a single-arm pivotal Phase II trial, and then expand into other new indications. GNTbm-TKI will be a multi-tyrosine kinase inhibitor with strong immunomodulatory effects, primarily applied in combination of 'GNTbm-TKI plus I' for indication expansion, where I represents HDAC inhibitor, ICI, or anti-PD-1/VEGF bispecific antibody, belonging to powerful cancer immunotherapy treatments that are expected to treat various advanced solid tumors.

The first indication of GNTbm-TKI --- neuroendocrine tumors
Neuroendocrine tumors (NETs) are a type of rare tumor that commonly occur in the neuroendocrine cells of the human body. These cells possess characteristics of both nerve cells and hormone-secreting endocrine cells. Most neuroendocrine tumors are malignant. NETs most commonly occur in the gastrointestinal tract, with approximately 20% occurring in the colon, 19% in the small intestine, and 4% in the appendix. The second most common site for NETs is the lungs, with about 30% of cases possibly occurring in the bronchial system. NETs of the gastrointestinal tract and lungs are both referred to as carcinoids.
In addition, approximately 7% of NETs occur in the pancreas, a condition known as pancreatic NET (pNET) or pancreatic islet cell tumor. Common symptoms of NETs or islet cell tumors include unexplained weight gain or loss, abdominal pain, vomiting blood, anxiety, sweating, headaches, loss of consciousness, inflammation of the tongue and mouth, abdominal lumps or masses, seizures, blurred vision, rapid heart rate, and jaundice.
According to statistics from Datamonitor Healthcare, the number of global NET cases in 2024 is approximately 588,200, and it is projected to increase to 679,400 by 2031. It is estimated that Asia will have the highest number of NET cases in 2024 (357,600 cases). According to Global Market Insights report, the incidence of NETs continues to rise in North America, Asia, and Europe, with the most significant increase observed in North America. For example, in the United States, the incidence of NETs has grown more than sixfold over the past 40 years, with the highest incidence rate in localized NET tumors.
In April 2025, Cabozantinib was approved by the U.S. FDA for the treatment of locally advanced or metastatic, previously treated, unresectable well-differentiated pancreatic neuroendocrine tumors (pNETs) and extra-pancreatic neuroendocrine tumors (epNETs). The targets inhibited by Cabozantinib are similar to those of Zanzalintinib and GNTbm-TKI (TAM, VEGFR2, c-MET), primarily inhibiting tumor growth and development, tumor invasion and metastasis, tumor angiogenesis, and immune regulatory activity. Compared to Cabozantinib and Zanzalintinib, the main distinction of GNTbm-TKI is its stronger inhibition of ROS1, BTK, NRTK2, and Tie2, along with more pronounced immune activation.
In addition, approximately 7% of NETs occur in the pancreas, a condition known as pancreatic NET (pNET) or pancreatic islet cell tumor. Common symptoms of NETs or islet cell tumors include unexplained weight gain or loss, abdominal pain, vomiting blood, anxiety, sweating, headaches, loss of consciousness, inflammation of the tongue and mouth, abdominal lumps or masses, seizures, blurred vision, rapid heart rate, and jaundice.
According to statistics from Datamonitor Healthcare, the number of global NET cases in 2024 is approximately 588,200, and it is projected to increase to 679,400 by 2031. It is estimated that Asia will have the highest number of NET cases in 2024 (357,600 cases). According to Global Market Insights report, the incidence of NETs continues to rise in North America, Asia, and Europe, with the most significant increase observed in North America. For example, in the United States, the incidence of NETs has grown more than sixfold over the past 40 years, with the highest incidence rate in localized NET tumors.
In April 2025, Cabozantinib was approved by the U.S. FDA for the treatment of locally advanced or metastatic, previously treated, unresectable well-differentiated pancreatic neuroendocrine tumors (pNETs) and extra-pancreatic neuroendocrine tumors (epNETs). The targets inhibited by Cabozantinib are similar to those of Zanzalintinib and GNTbm-TKI (TAM, VEGFR2, c-MET), primarily inhibiting tumor growth and development, tumor invasion and metastasis, tumor angiogenesis, and immune regulatory activity. Compared to Cabozantinib and Zanzalintinib, the main distinction of GNTbm-TKI is its stronger inhibition of ROS1, BTK, NRTK2, and Tie2, along with more pronounced immune activation.
The development of GNTbm-TKI's new indications adopt a basket trial strategy --- advanced kidney cancer, advanced head and neck cancer, advanced colorectal cancer
The combination of GNTbm-TKI + GNTbm-38 + anti-PD-1/VEGF bispecific antibody represents a potent cancer immunotherapy regimen. In animal experiments, we have verified that GNTbm-TKI + GNTbm-38 possesses excellent activity of tumor microenvironment modulation and immune-activating anti-cancer activity; furthermore, we also observed the remarkable anti-cancer activity of GNTbm-38 combined with anti-PD-1 antibody. Ultimately, using GNTbm-TKI + GNTbm-38 + anti-PD-1 antibody combination, we achieved the very outstanding anti-cancer efficacy in immuno-oncology. The anti-PD-1/VEGF bispecific antibody provides superior anti-cancer benefits compared to individual anti-PD-1 antibody and Bevacizumab combination. Therefore, we recommend the combination therapy of GNTbm-TKI + GNTbm-38 + anti-PD-1/VEGF bispecific antibody for the treatment of various advanced cancers, including first-line treatment for advanced renal cancer, first-line treatment for advanced head and neck cancer, and second-line treatment for advanced colorectal cancer.
For the combination of GNTbm-TKI + GNTbm-38 + anti-PD-1/VEGF bispecific antibody, it is suitable for us to adopt a basket development strategy, enrolling 20 patients for each indication to evaluate efficacy and safety. If the primary efficacy endpoints of the trial are met, we will further plan to enter a pivotal Phase III clinical trial, with a strategy to enroll patients in multiple countries and centers, mainly in the United States, the European Union, Canada, Australia, Taiwan, China, and Japan, to further verify efficacy and safety.
For the combination of GNTbm-TKI + GNTbm-38 + anti-PD-1/VEGF bispecific antibody, it is suitable for us to adopt a basket development strategy, enrolling 20 patients for each indication to evaluate efficacy and safety. If the primary efficacy endpoints of the trial are met, we will further plan to enter a pivotal Phase III clinical trial, with a strategy to enroll patients in multiple countries and centers, mainly in the United States, the European Union, Canada, Australia, Taiwan, China, and Japan, to further verify efficacy and safety.