Epigenetic Immunoactivator
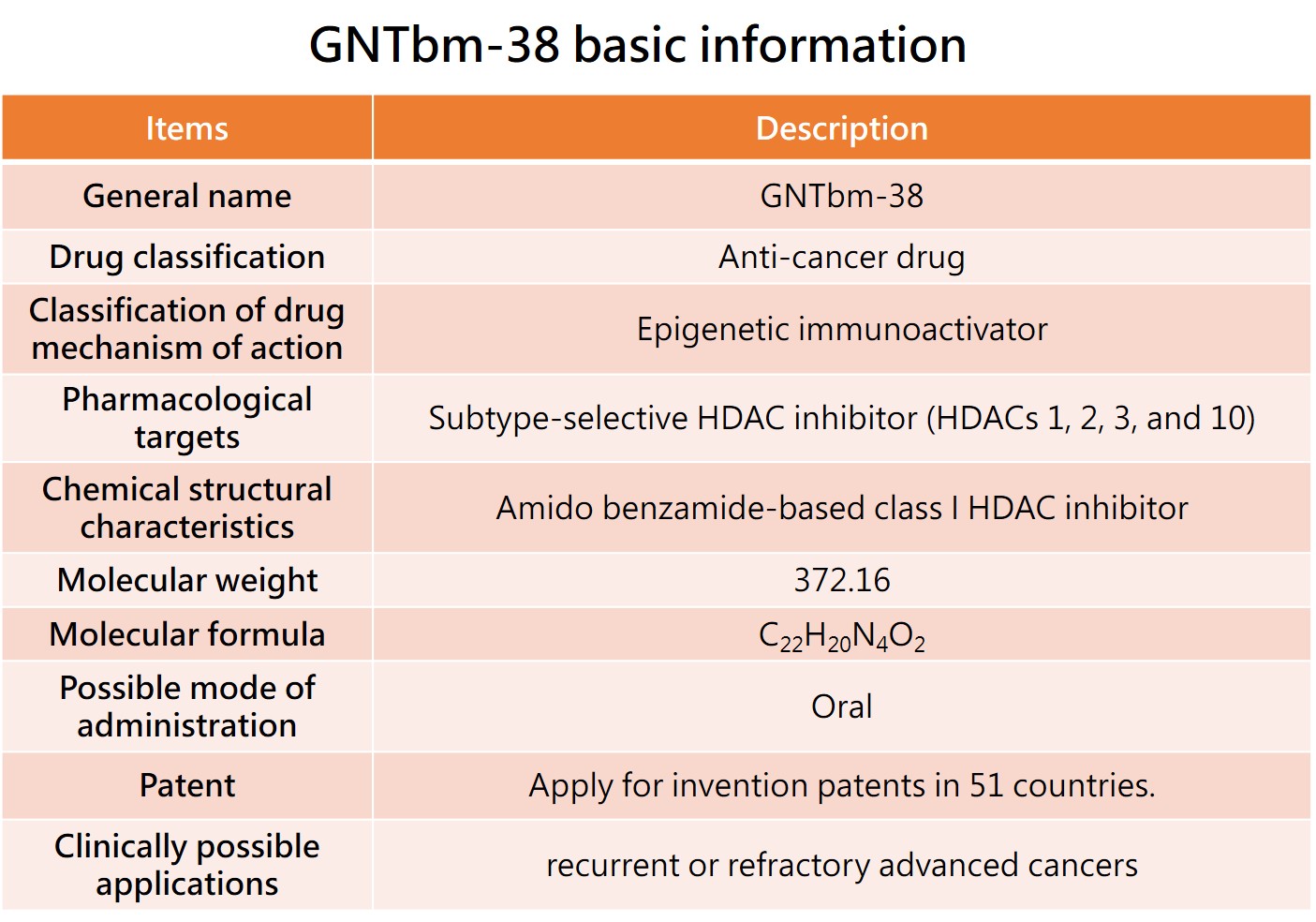
The GNTbm R&D team has independently developed a new structural epigenetic immunoactivator, named GNTbm-38, after years of R&D. It is a dual-function drug that has been screened for innovative, small-molecule, oral, and excellent epigenetic regulation and immune modulation activities. Studies on enzyme, cell, and animal test platforms have shown that GNTbm-38 has excellent epigenetic and immune modulation activities. When used in combination with TKIs that inhibit specific targets, it demonstrates very superior activity in remodeling the TME, significantly increasing the tumor response rate. GNTbm-38 can be used as a monotherapy or in combination with TKIs that inhibit specific targets for cancer immunotherapy. The chemical structure of GNTbm-38 falls under the category of benzamide-based, serving as a selective class I HDAC inhibitor. GNTbm has obtained multiple invention patents, including in the United States. GNTbm-38 is one of the essential components in cancer combination immunotherapy.
The GNTbm-38 pre-clinical studies have been successfully completed, and an IND will be applied to conduct a Phase 1 clinical trial, followed by a Phase 2 clinical trial, with R/R PTCL as the first indication. An application for orphan drug designation in the United States will be submitted. Once the R/R PTCL drug approval is obtained, new indications will be expanded, by using 'GNTbm-38 + Z’, where Z could be a TKI that inhibits unique targets or a anti-PD-1/VEGF bispecific antibody, for treatment of various advanced solid tumors.
The first indication of GNTbm-38---R/R PTCL
Treatment of T-cell lymphomas is more challenging compared to B-cell lymphomas, with no standard treatment regimen. The numerous subtypes, high heterogeneity and significant disease severity have resulted in an urgent unmet clinical need. GNTbm-38 has been demonstrated its efficacy by using a mouse R/R AITL PDX model. Compared to the positive control group treated with Belinostat injections, the oral administration of GNTbm-38 showed superior efficacy, once again confirming that GNTbm-38 as a monotherapy is applicable for treatment of R/R PTCL.
GNTbm will conduct a dose escalation study of GNTbm-38 in clinical Phase Ia by enrolling patients with advanced solid tumors. After further confirming DLT/MTD and the optimal recommended dose, it will proceed to a Phase Ib trial, enrolling R/R PTCL patients in multiple countries for efficacy and safety studies. If the trial results meet expectations, these clinical trial results will be used to apply for orphan drug designation of GNTbm-38 for the treatment of R/R PTCL in the United States, the European Union, Japan, and China.
After receiving orphan drug designation in multiple countries, GNTbm-38 will be filed for fast-track review status, with a pivotal Phase II trial enrolling patients in the United States, Canada, Australia, Europe, Japan, Taiwan, and China. If the efficacy and safety meet expectations, NDA applications will be submitted in multiple countries in the hope of providing new treatment option for patients with R/R PTCL.
New indication for GNTbm-38 --- MYC/BCL2 double-expressor DLBCL (MYC/BCL2 DE-DLBCL)
In 2024, Tucidinostat combined with R-CHOP significantly increased the CR rate of first-line treatment in patients with MYC/BCL2 DE-DLBCL, rising from 61.8% to 73%. The primary efficacy endpoint, EFS, also showed a statistically significant difference, leading to the approval of a new indication of Tucidinostat in China, benefiting patients with MYC/BCL2 DE-DLBCL.
However, GNTbm has been continuously thinking how to further increase the CR rate up to 85%. Compared to Tucidinostat, GNTbm-38 has better anti-cancer activity. For patients with MYC/BCL2 DE-DLBCL, only achieving CR can guarantee to extend their PFS and OS. For this reason, we hope to strengthen the treatment regimen by combining GNTbm-38 + BTK inhibitor + Rituximab ± CHOP. If a patient achieves CR in the first two treatment cycles with the GNTbm-38 + BTK inhibitor + Rituximab combination, they will continue for 6 more treatment cycles. If a patient only achieves PR or SD in the first two treatment cycles with the GNTbm-38 + BTK inhibitor + Rituximab combination, the patient will need to receive 6 treatment cycles of the combined CHOP chemotherapy regimen to convert from PR/SD to CR.
MYC is an oncogene that promotes cancer cell proliferation. High MYC protein expression makes DLBCL more resistant to killing. If accompanied by high BCL2 protein expression, it is even harder to induce cancer cell apoptosis. High expression of MYC/BCL2 can make it difficult for standard R-CHOP treatment to achieve complete remission (CR). According to WHO, MYC protein expression above 40% and BCL2 protein expression above 50% are considered high expression.
New indication for GNTbm-38 —MSS/pMMR mCRC
Late-stage colorectal cancer is no longer operable and requires systemic therapy. Since 90% of patients are of the MSS/pMMR type, they cannot be treated with current cancer immunotherapies. However, this impasse was broken in April 2024 with the combination of Tucidinostat + anti-PD-1 antibody + Bevacizumab. This triple drug combination effectively remodels the tumor microenvironment, transforming mCRC, which originally belonged to cold tumors, into hot tumors, significantly improving ORR to 44% and achieving a historic mPFS of 7.3 months.
The triple drug combination of Tucidinostat + anti-PD-1 antibody + Bevacizumab has already entered a pivotal Phase III trial to evaluate its efficacy and safety, with fruquintinib as the control group. The primary efficacy endpoint of the clinical trial is median overall survival (mOS), and it is expected to achieve positive results.
GNTbm-38 is an epigenetic regulator with better tumor immune activation properties compared to Tucidinostat. It will be tested in combination with an anti-PD-1/VEGF bispecific antibody for third-line treatment of advanced colorectal cancer, by enrolling MSS/pMMR patients for proof of concept (POC). GNTbm-38 provides a unique pharmacological contribution to immune activation. When combined with an anti-PD-1/VEGF bispecific antibody, it is expected to offer better therapeutic benefits and lower side effects compared to combination with individual anti-PD-1 antibodies and Bevacizumab. This also represents the most important POC validation for GNTbm-38 in cold tumors of advanced solid tumors.