Uncovering the anti-cancer mechanisms of Immuno-Oncology
Tumor Microenvironment Regulators
1. GNTbm-CC-01(CC-01)
In the tumor microenvironment, GNTbm-CC-01 significantly reduces the tumor-infiltrating Treg cells (regulatory T cells), Besides, we also found both numbers of tumor-infiltrating PMN-MDSCs (polymorphonuclear myeloid-derived suppressor cells) and M-MDSCs were significantly decreased. On the other hand, the large number of TAMs (tumor-associated macrophages) also significantly declined. However, the numbers of tumor-infiltrating CD4+T cells and CD8+T cells were not significantly changed.
GNTbm-CC-01 has potent anti-cancer immune response activity, mainly reduces the number and function of many immunosuppressive cells and activates the activity of CD8+T cells and NK cells to kill tumors. It will eventually produces memory T cells, effectively surveilling and killing cancer cells expressing the same antigen for T cell recognition, and therefore producing permanent immune activity. The combination of GNTbm-CC-01 with immune checkpoint inhibitor will further enhance the anti-cancer effect and boost the immune response rate.
GNTbm-CC-01 patent has been submitted to 23 regions or countries.
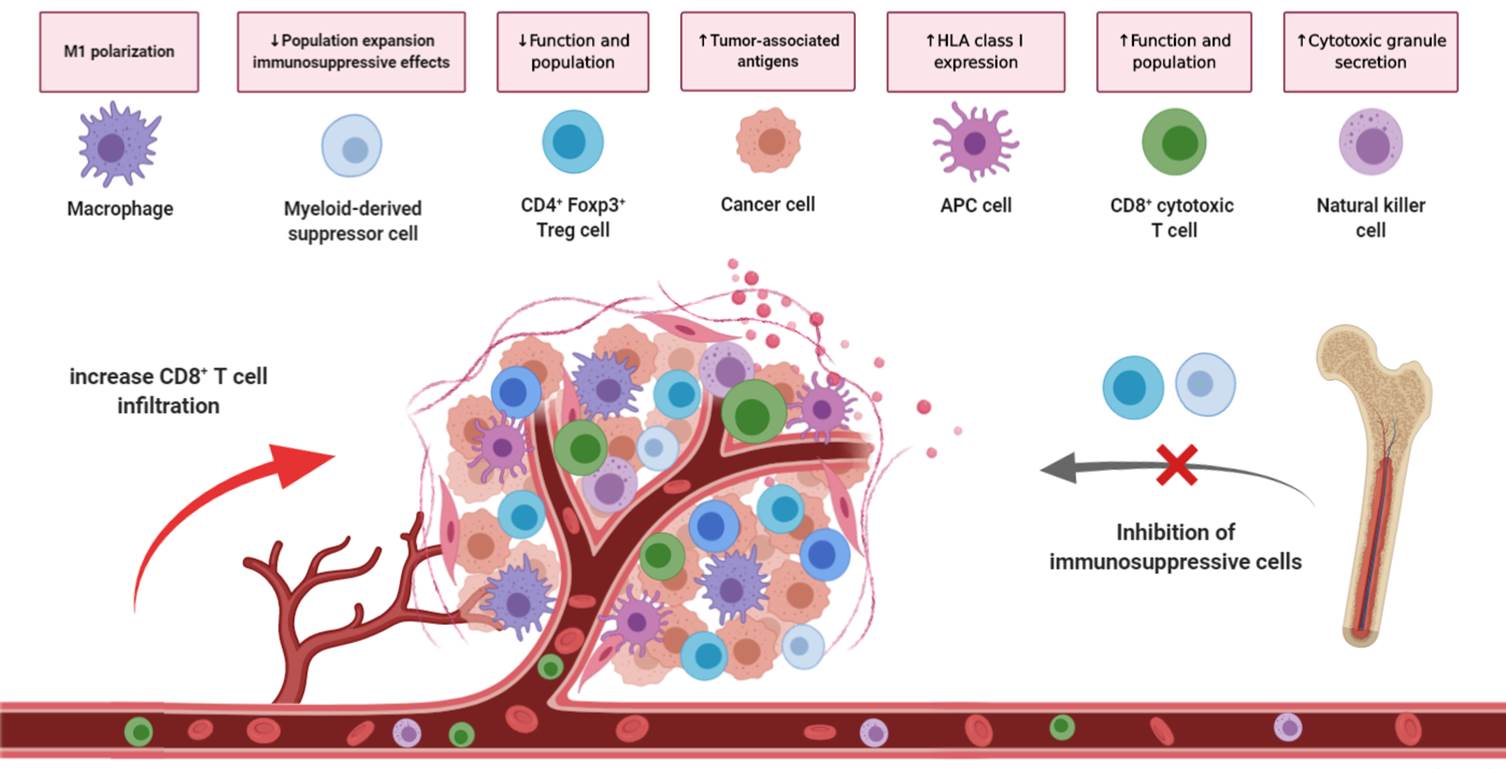
Figure 1. Effects of GNTbm-CC-01 in the Tumor Microenvironment.
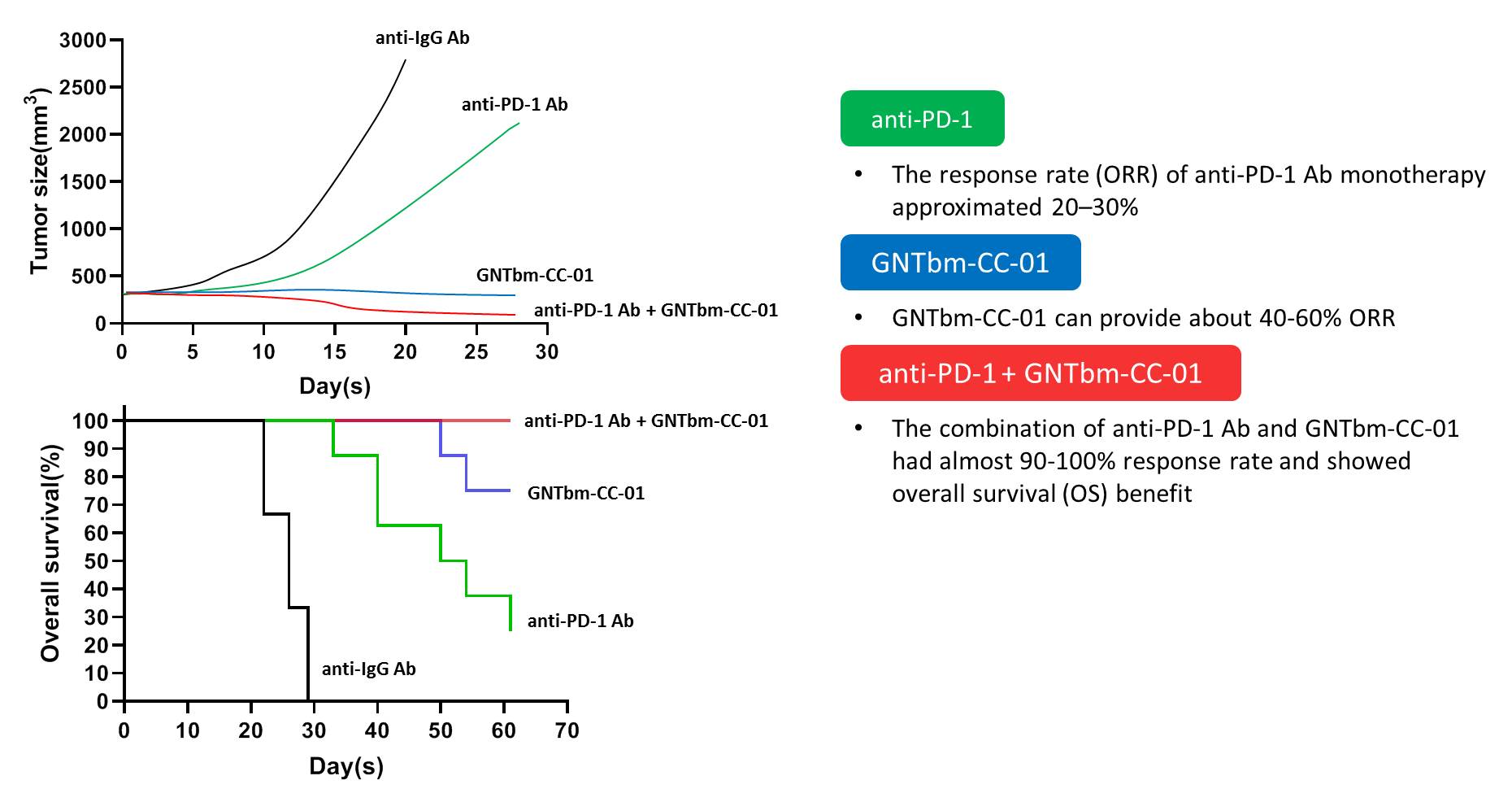
Figure 2. Efficacy of GNTbm-CC-01 in the Tumor Microenvironment.
2. GNTbm-CC-02
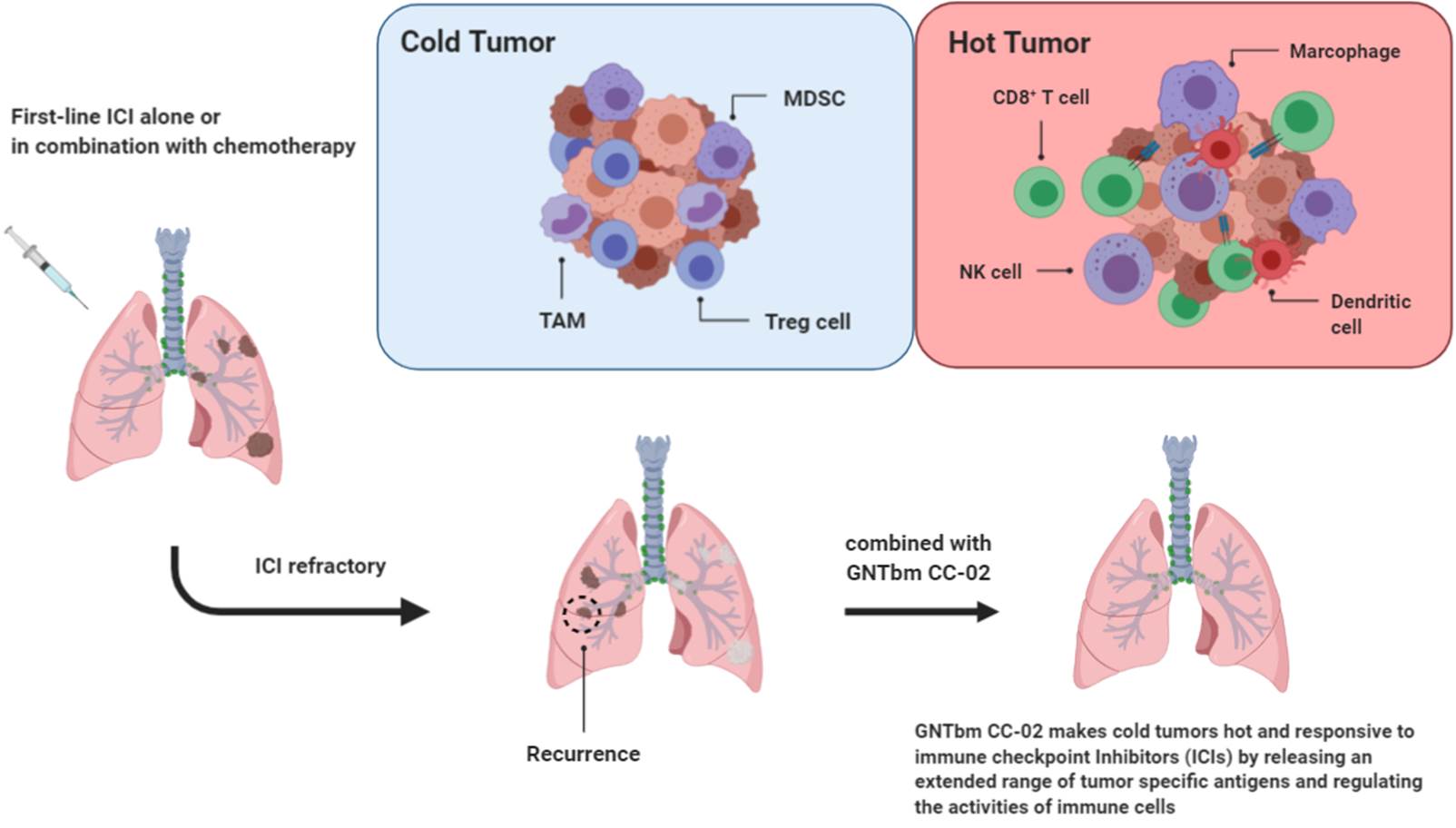
CC-02 overcomes the drug resistance issue caused by the first-line anti-PD-1 Ab treatment
CC-02 controlled the tumor microenvironment more efficiently with enhanced inhibition of immunosuppressive cells homing to the tumor site. These make CC-02 a powerful regimen to overcome the drug resistance issue caused by the first-line immune checkpoint inhibitor therapy or HPD (Hyperprogressive disease). Therefore, CC-02 will play an important role in the combination therapy of immune checkpoint inhibitors against drug resistance.
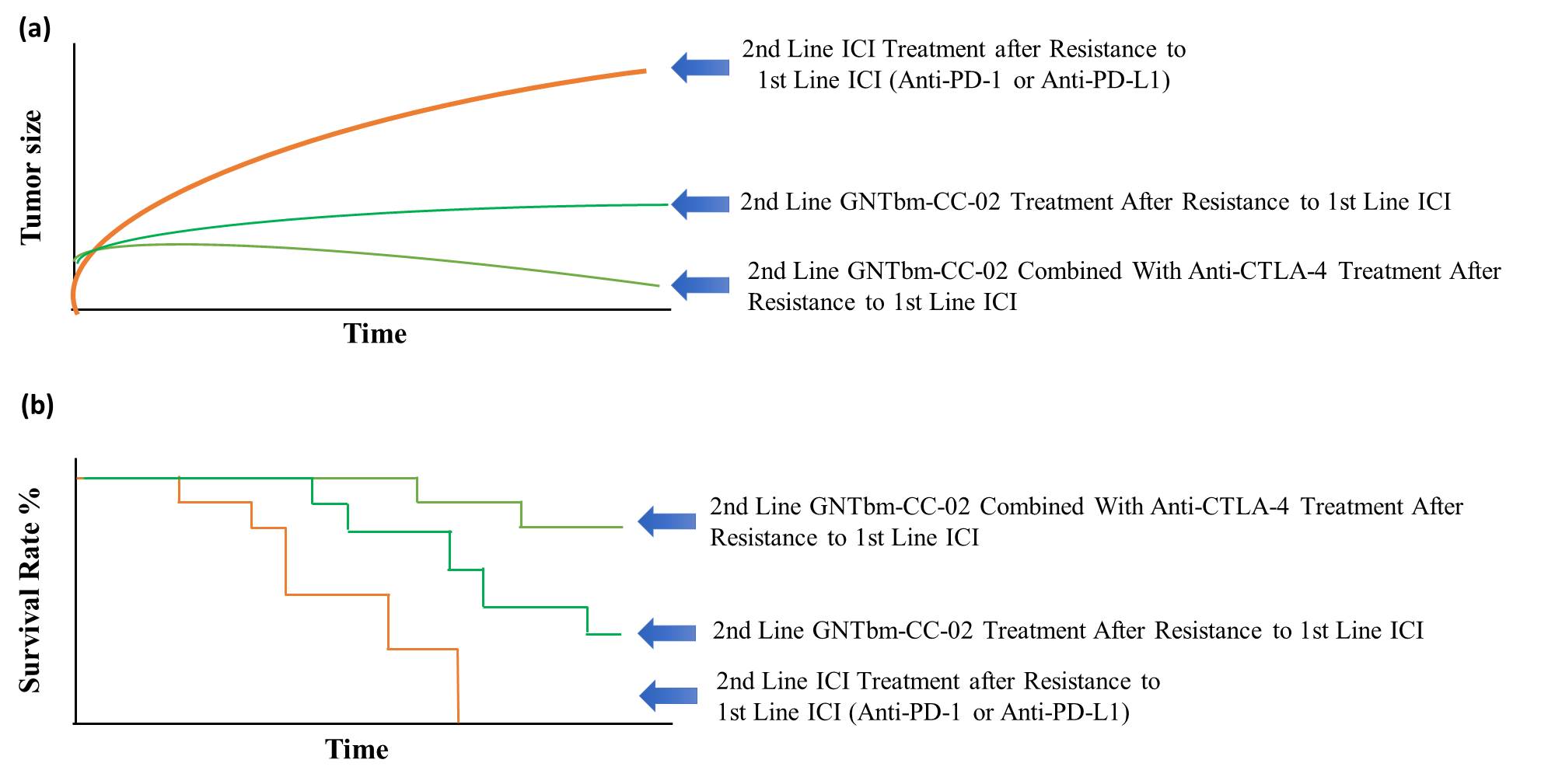
Figure 4. Efficacy of GNTbm-CC-02 Treatment after Resistance to First Line Anti-PD-1/Anti-PD-L1. (a) Inhibition of tumor growth by GNTbm-CC-02 or in combination with anti-CTLA-4 Ab (b) Survival rate.
Figure 5. GNTbm-CC-02 as Tumor Microenvironment Regulator Can Overcome Drug Resistance Caused by the First-Line ICI Treatment.
3. GNTbm-CT-01
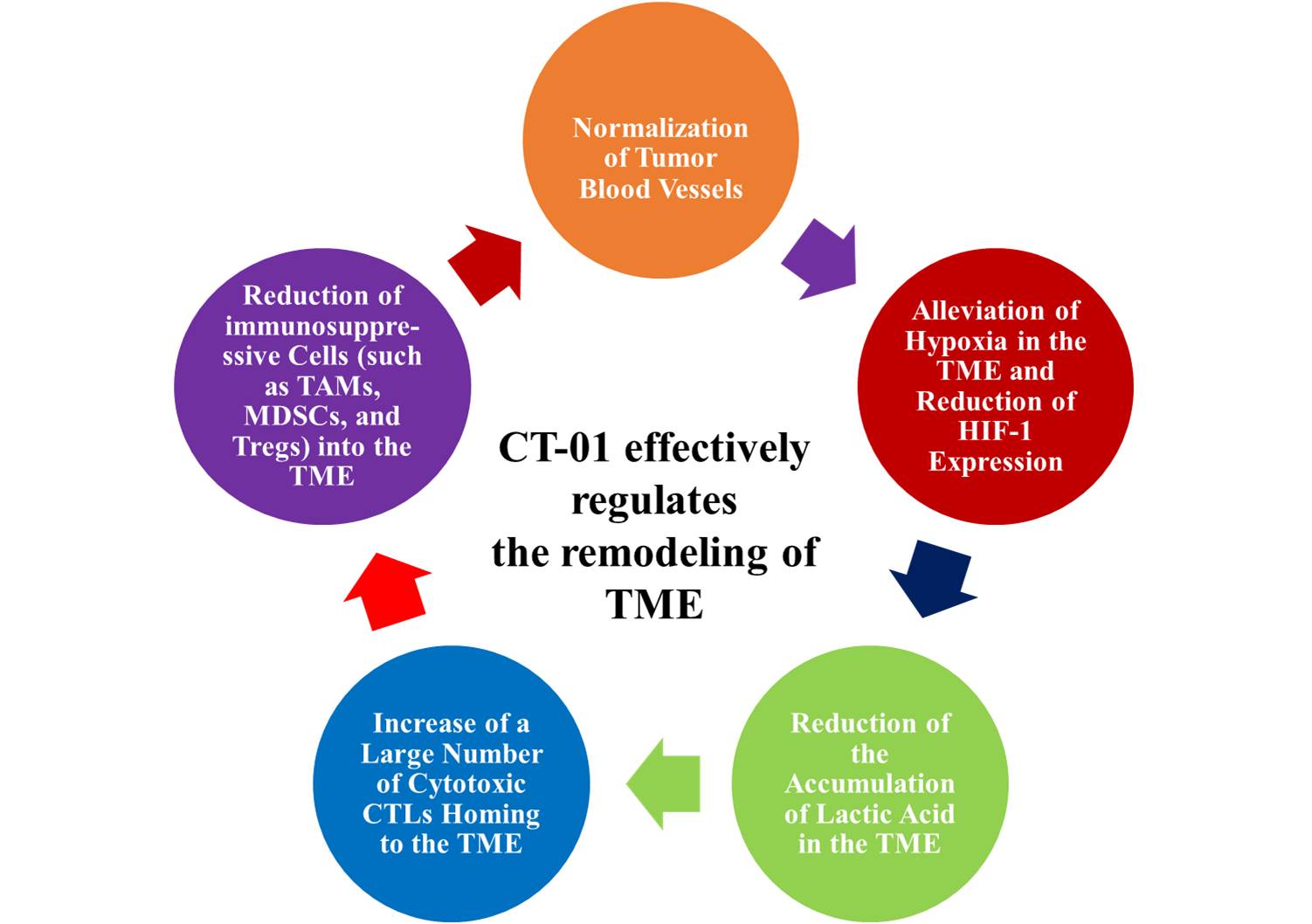
CT-01 is the first powerful tumor microenvironment modulator (TMR) independently developed by GNTbm that does not require combination with immune checkpoint inhibitor. CT-01 is composed of two already marketed oral drugs, namely Tucidinostat and Regorafenib, through the unique tumor microenvironment regulation mechanism of these two drugs, remodel the tumor microenvironment, normalize tumor blood vessels (a key step in remodeling the tumor microenvironment), alleviate the hypoxic state of the tumor microenvironment, reduce the accumulation of lactic acid, and induce a large number of activated CTL homing to the tumor microenvironment. It effectively prevents the infiltration of immunosuppressive cells (such as TAMs, MDSCs, and Tregs) into the tumor microenvironment, and can significantly regulate the gene expression of cytokines and chemokines in the tumor microenvironment, which is conducive to killing of cancer cells and achieving excellent anti-cancer effects. On the other hand, it is conducive to regulating progenitor cells in the bone marrow microenvironment, reducing the differentiation of immunosuppressive cells and homing into the tumor microenvironment, which will have positive effect for the eradiation of tumors by inducing long-term immune memory to prevent tumor recurrence.
CT-01 is a powerful TMR independently developed by the R&D team of GNTbm. The first human clinical trial for POC is for second-line treatment in intermediate and advanced HCC patients. In hope, TMR CT-01 with innovative anti-cancer mechanism will meet the medical needs for the treatment of patients with intermediate and advanced HCC.
If CT-01 is successful in POC for intermediate and advanced HCC, GNTbm will expand to other new indications to provide better treatment benefits and new options for more advanced cancer patients.