Tucidinostat/Chidamide (Trade name in Mandarin :剋必達®, Trade name in English : Kepida®)
Tucidinostat's first indication in Taiwan – combined with Exemestane for the treatment of HR+/Her-2-advanced breast cancer
Kepida® has obtained the drug license for domestically developed new new drug after 10 years of development in Taiwan
The trade name of Tucidinostat in Taiwan is Kepida®, a new generation oral epigenetic modulator. It is a subtype-selective HDAC inhibitor, and GNTbm spent 10 years and invested hundreds of millions in research and development to obtain the domestic certification for a new drug.

Pivotal Phase III Clinical Trial for the Tucidinostat Treatment of HR+/HER-2- Advanced Breast Cancer (CDM301)
GNTbm submitted the clinical trial protocol for Phase III to treat advanced breast cancer to the TFDA/CDE for review and received recommendations from TFDA/CDE. The trial design and inclusion criteria for patients were the same as that of the clinical trial conducted in China, and it was suggested that the clinical data from Taiwan were combined with the clinical data from China to serve as the endpoints for this trial.
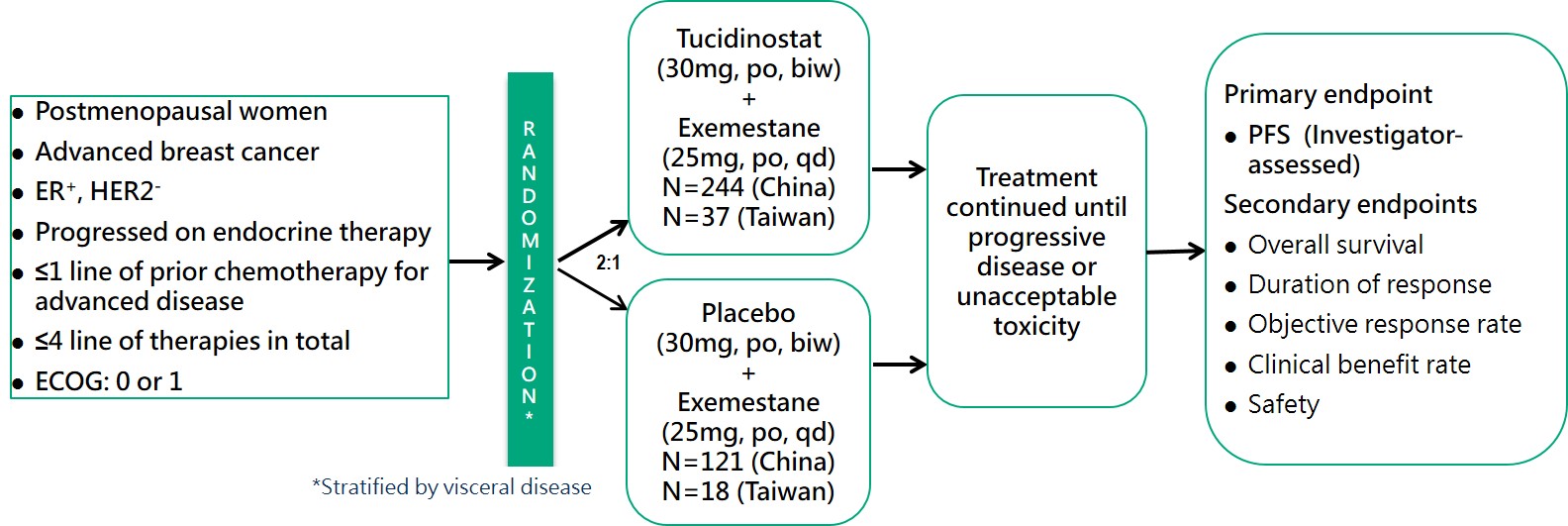
The clinical trial, called CDM301, recruited patients with HR+/Her-2- advanced breast cancer who had failed or relapsed with at least one previous endocrine therapy, treated with Tucidinostat plus Exemestane. The patients were stratified according to presence or absence of visceral metastasis, and randomly assigned 2:1 to the experimental group and the placebo group for treatment until disease progression or withdrawal from the trial due to drug toxicity, and the primary endpoint was PFS assessed by clinical investigators. The trial period was about 5 years.
Efficacy assessment
CDM301 is a well-designed, placebo-controlled, randomized, multicenter, pivotal Phase III clinical trial in Taiwan and China. A total of 420 patients were enrolled and randomized 2:1 to Tucidinostat plus Exemestane (experimental group) and placebo plus Exemestane (placebo group), with 281 and 139 patients, respectively. The efficacy outcomes mPFS of the primary endpoint of the trial were 7.4 months and 3.7 months (HR=0.716; 95% CI, 0.562 ~ 0.911; P=0.0066) for the experimental and placebo groups, respectively. Data analysis of 55 patients in Taiwan showed that Tucidinostat combined with Exemestane (experimental group) and placebo plus Exemestane (placebo group) were randomly assigned 2:1, with 37 and 18 cases, respectively. The mPFS of the primary endpoint were 8.6 months and 3.7 months (HR=0.516; 95% CI, 0.268 ~ 0.993; P=0.0477), for the experimental and placebo groups, respectively. In 420 patients, the objective response rate (ORR) of the secondary endpoint efficacy of the trial was 16.73% in the experimental group and 7.91% in the placebo group, which showed a statistically significant difference.
Safety data
Common adverse events in the Tucidinostat combined with Exemestane groups were hematologic toxicity and gastrointestinal toxicity, both of which were controllable adverse reactions that could be restored by reducing the dose of investigational drug or suspending the investigational drug.
Tucidinostat meets the medical needs of HR+/Her-2- advanced breast cancer patients as a second-line treatment
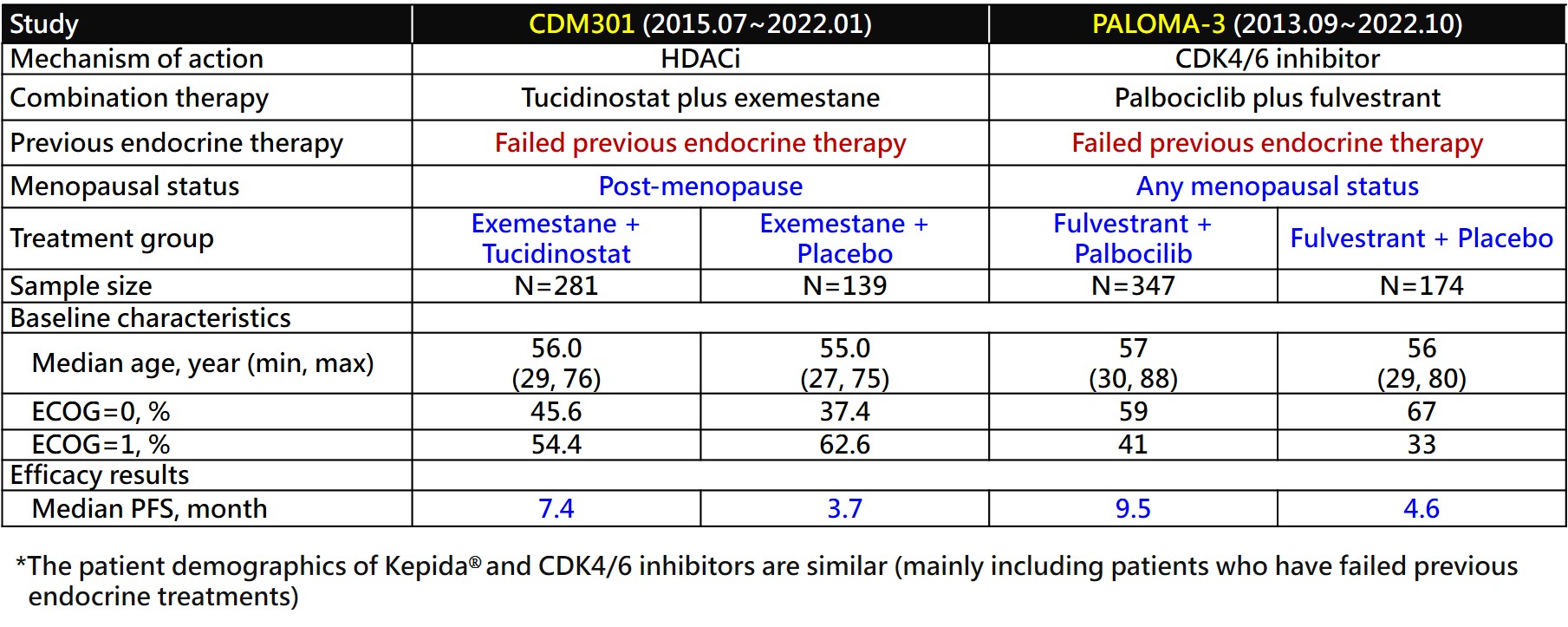
According to the NCCN treatment guidelines and the expert consensus of the Taiwan Breast Cancer Society, the first-line treatment for HR+/Her-2- advanced breast cancer primarily recommends endocrine therapy combined with CDK4/6 inhibitor as the standard treatment. Additionally, based on Taiwan's National Health Insurance reimbursement regulations, HR+/Her-2- advanced breast cancer patients can receive CDK4/6 inhibitors for 24 months with health insurance coverage throughout their lifetime. Furthermore, international literature reports that the first-line treatment for HR+/Her-2- advanced breast cancer using endocrine therapy combined with CDK4/6 inhibitor has shown excellent treatment efficacy, with a median progression-free survival (mPFS) of about 24 months. These reasons support that approximately 70% of HR+/Her-2- advanced breast cancer patients in Taiwan will use endocrine therapy combined with CDK4/6 inhibitor in the first-line treatment.
When the first-line treatment using endocrine therapy combined with CDK4/6 inhibitor fails, it can result in patients having dual resistance to both endocrine therapy and CDK4/6 inhibitor. Consequently, the options for subsequent second-line treatment will be more limited, primarily due to the occurrence of dual resistance after first-line treatment in patients with advanced breast cancer. According to the NCCN treatment guidelines, the medications available for second-line treatment are quite limited. The first recommendation: if the patient has never used a CDK4/6 inhibitor, the second-line treatment can recommend using a CDK4/6 inhibitor combined with endocrine therapy; the second recommendation: if the patient has a PIK3CA mutation, Alpelisib (PI3Ki) combined with fulvestrant can be recommended; the third recommendation is that the patient may use Everolimus (mTORi) combined with Exemestane.
Tucidinostat (HDACi) in combination with Exemestane will provide an alternative choice. Tucidinostat is an epigenetic regulator that primarily regulates gene expression at a deeper level, which is significantly different from the mechanisms of action of currently approved drugs. Mechanistically, it can address the dual resistance issues caused by long-term treatment with endocrine therapy and CDK4/6 inhibitor. GNTbm will conduct a real-world study in Taiwan to evaluate the efficacy of Tucidinostat combined with Exemestane in the second-line treatment after failure of first-line treatment with endocrine therapy in combination with CDK4/6 inhibitor for HR+/Her-2- advanced breast cancer.
Tucidinostat may resolve the dual resistance problem arising from the first-line treatment with endocrine therapy combined with CDK4/6 inhibitor for HR+/Her-2- advanced breast cancer
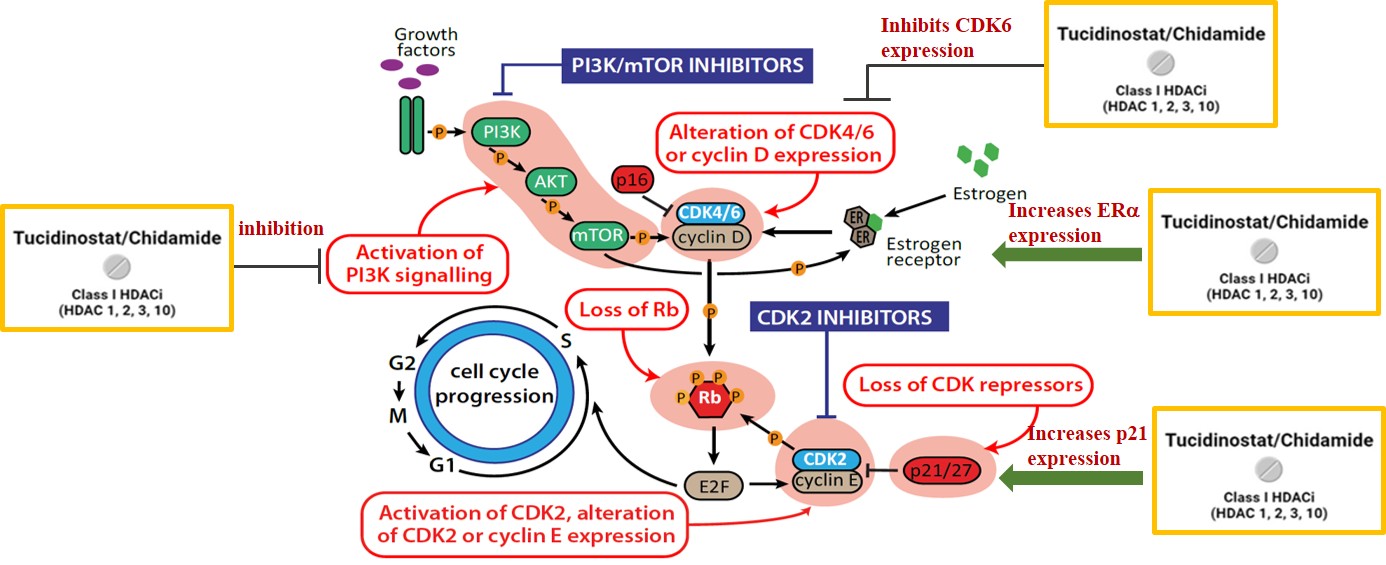
Kepida®'s different new mechanisms of action offer a new option for second-line treatment of advanced breast cancer
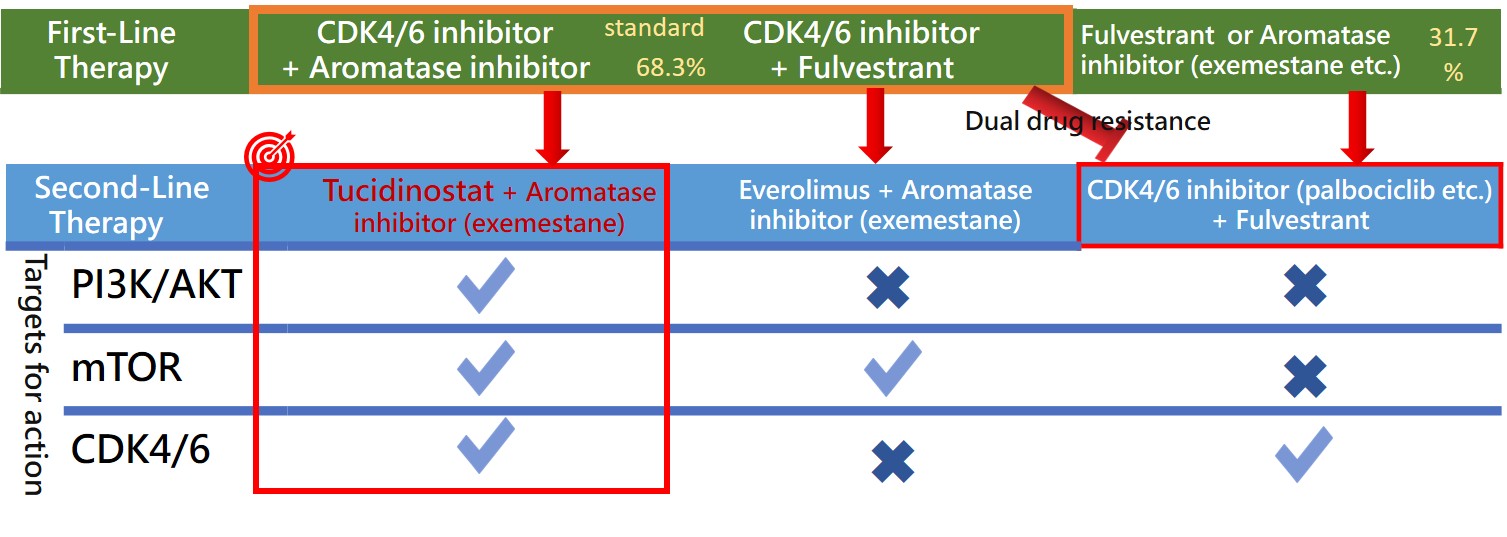
Clinical efficacy comparison: Kepida® is on par with CDK4/6 inhibitors