Tucidinostat/Chidamide (Trade name in Mandarin :剋必達®, Trade name in English : Kepida®)
Anti-cancer Mechanism of Tucidinostat
Tucidinostat is a benzamide-based histone deacetylase inhibitor (HDACi). Under physiological concentration in patients, it exerts significant inhibition on the enzymatic activities of HDAC subtypes 1, 2, 3, and 10, but not on the activities of other subtypes.
Studies have shown that chidamide exerts its anti-cancer effects through various mechanisms, including inhibition of the cancer cell cycle, induction of cancer cell differentiation and apoptosis, regulation of anti-cancer immunity, and inhibition of EMT.
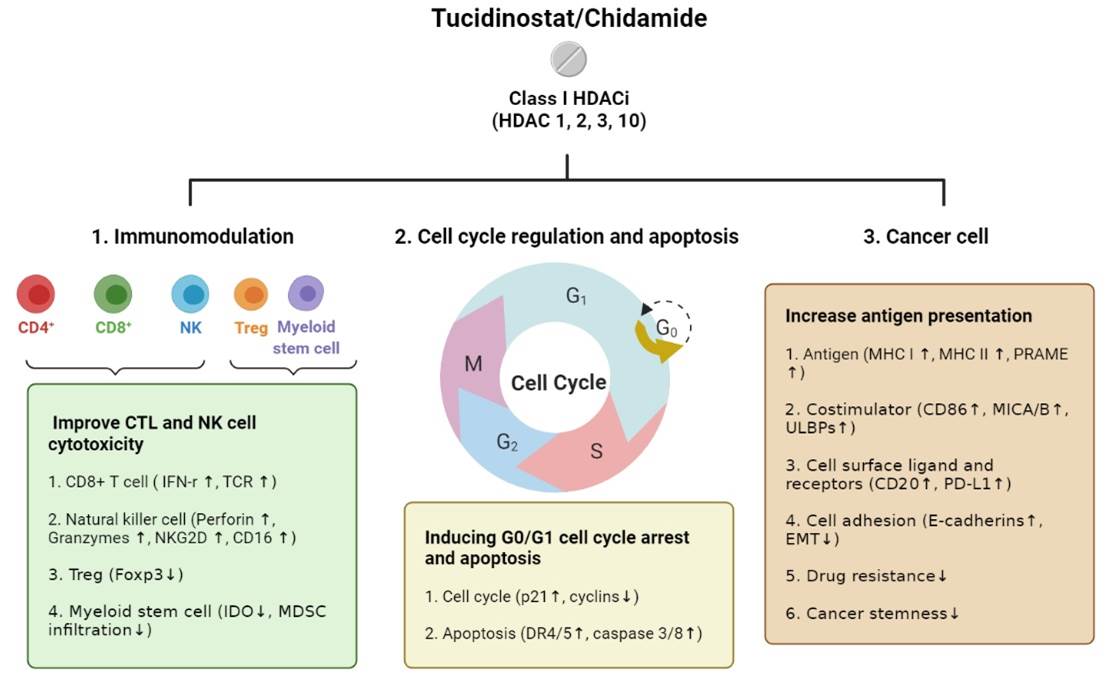
Figure 1. Tucidinostat possible anti-cancer mechanism.